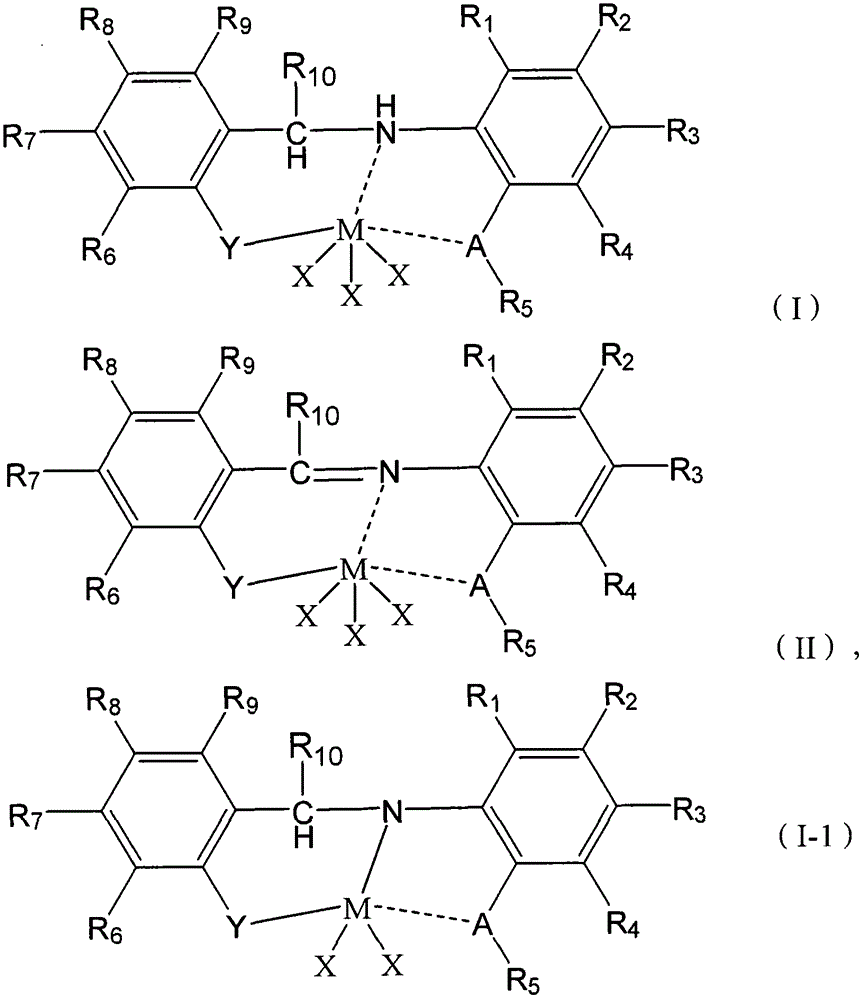
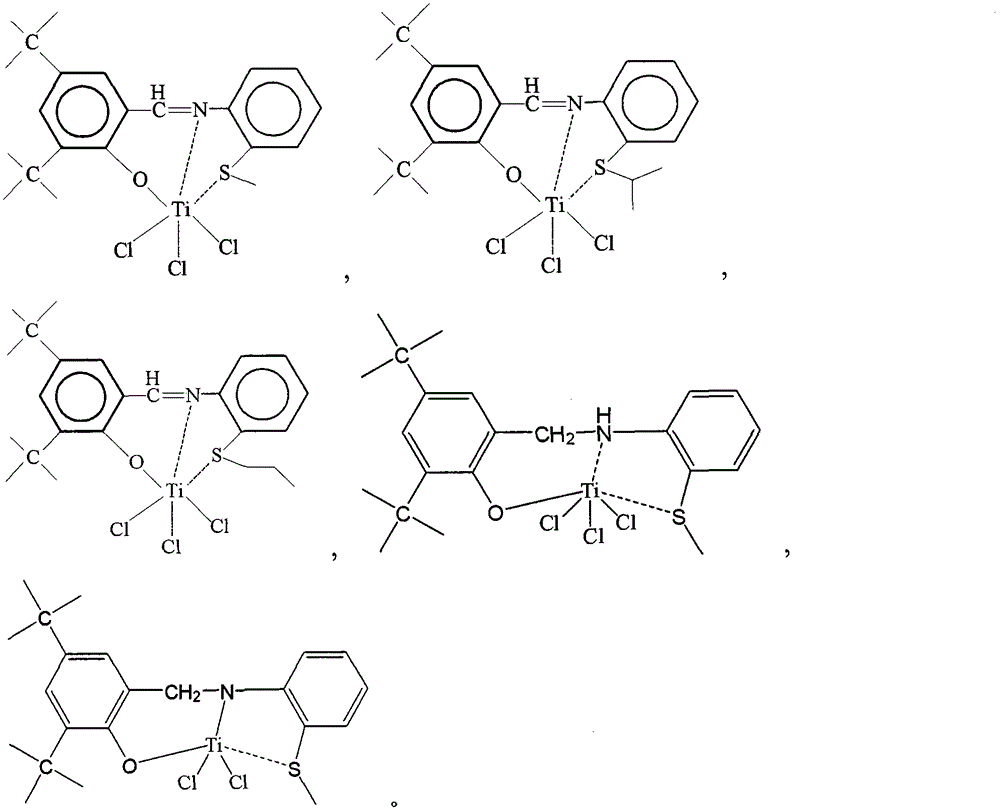
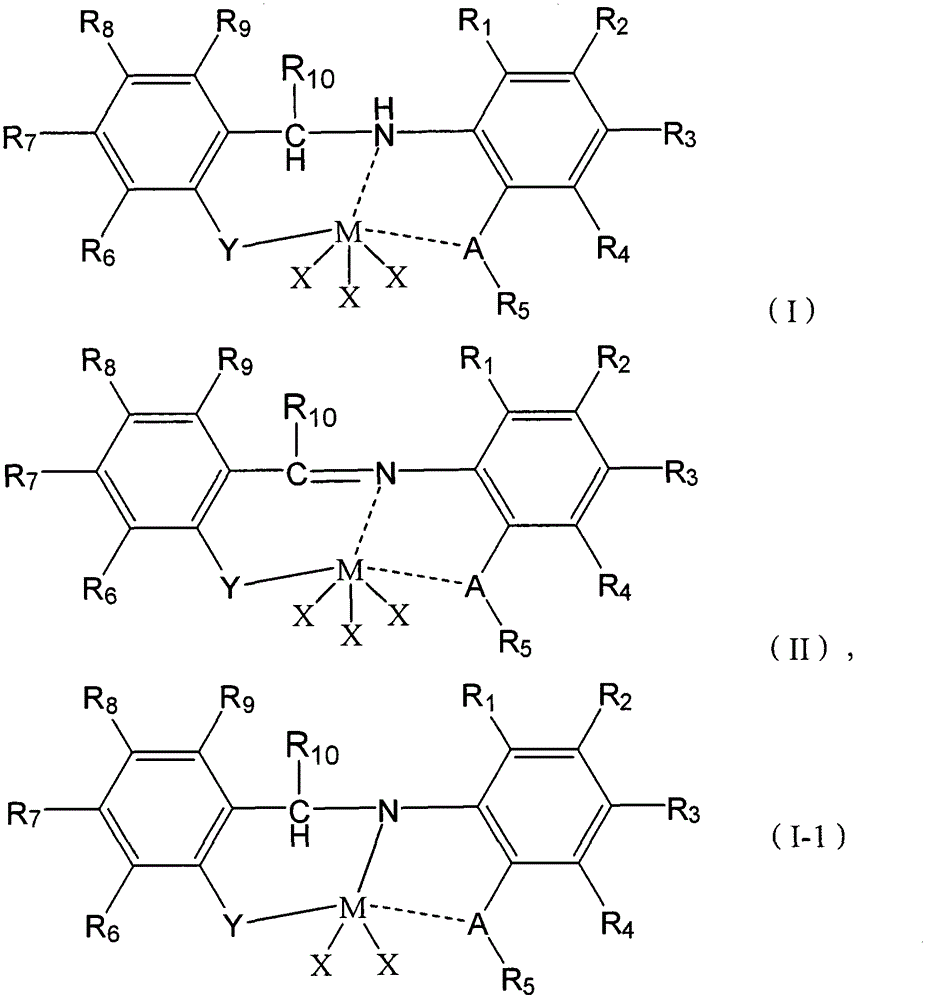
Cocatalyst composition and application thereof
A co-catalyst and composition technology, applied in the field of copolymerization of alkenes and cyclic olefins, can solve the problem of unsatisfactory glass transition temperature of copolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0210] The following examples will be used to illustrate the present invention in further detail, but the present invention is not limited to these examples.
[0211] The various performance parameters involved in the following examples and comparative examples were measured according to the following methods.
[0212] (1) Polymerization activity
[0213] Catalyst polymerization activity (unit is g(P)·(mol(M)·h) -1 , where P refers to the copolymer, M refers to the metal element M such as Ti) is calculated according to the following formula:
[0214] Polymerization activity = m 1 ×4×Mw / m 2
[0215] in,
[0216] m 1 The amount (g) of copolymer obtained for 15 minutes of polymerization time;
[0217] Mw is the molecular weight of catalyst;
[0218] m 2 is the amount of catalyst added (g).
[0219] (2) Molecular weight Mη of polymer
[0220] The viscosity-average molecular weight Mη of polymer is calculated according to the following method:
[0221] According to the ...
manufacture Embodiment 1
[0228] In a dry 500ml three-necked flask, add 2-aminoanisole sulfide (0.2mol), absolute ethanol (160ml), 3,5-di-tert-butyl salicylaldehyde (0.2mol), acetic acid (0.3ml) successively , heated up to reflux temperature and reacted for 2hr, cooled to room temperature, filtered, washed three times with absolute ethanol, and dried in vacuum to obtain 49.7g of 2-aminoanisole sulfide 3,5-di-tert-butyl salicylaldehyde, called Ligand L1,
[0229]
[0230] L1.
[0231] Elemental analysis: C74.57% (theoretical value 74.32%); H8.35% (theoretical value 8.22%); N4.07% (theoretical value 3.94%). 1 H NMR δ=13.4(OH), 8.6(CHN), 7.5-7.1(Ar-H), 3.25(SCH 3 ), 1.45 (C (CH 3 ) 3 ), 1.35 (C (CH 3 ) 3 ).
manufacture Embodiment 2
[0233] In a dry 500ml three-necked flask, add 2-aminophenylpropyl sulfide (0.2mol), absolute ethanol (160ml), 3,5-di-tert-butyl salicylaldehyde (0.2mol), acetic acid (0.3ml) successively , heated up to reflux temperature and reacted for 2hr, cooled to room temperature, filtered, washed three times with absolute ethanol, and dried in vacuum to obtain 49.7g of 2-aminophenylpropyl sulfide 3,5-di-tert-butyl salicylaldehyde, called Ligand L2,
[0234]
[0235] L2.
[0236] Elemental analysis: C74.57% (theoretical value 75.15%); H8.35% (theoretical value 8.67%); N3.95% (theoretical value 3.65%). 1 H NMR δ=13.4(OH), 8.55(CHN), 7.5-7.1(Ar-H), 2.10(CH 2 CH 3 ), 1.47 (C (CH 3 ) 3 ), 1.35 (C (CH 3 ) 3 ), 1.17 (CH 2 CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com