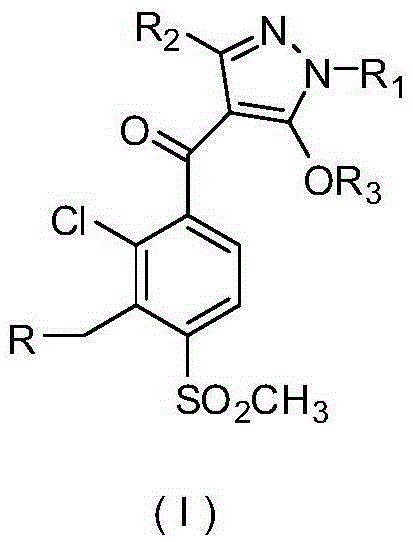
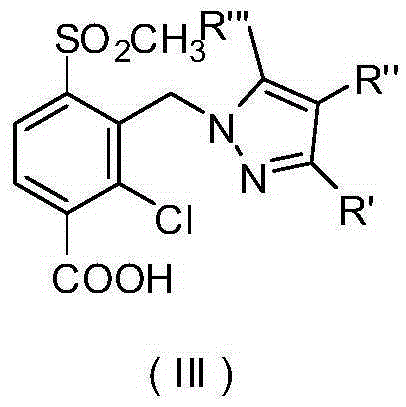
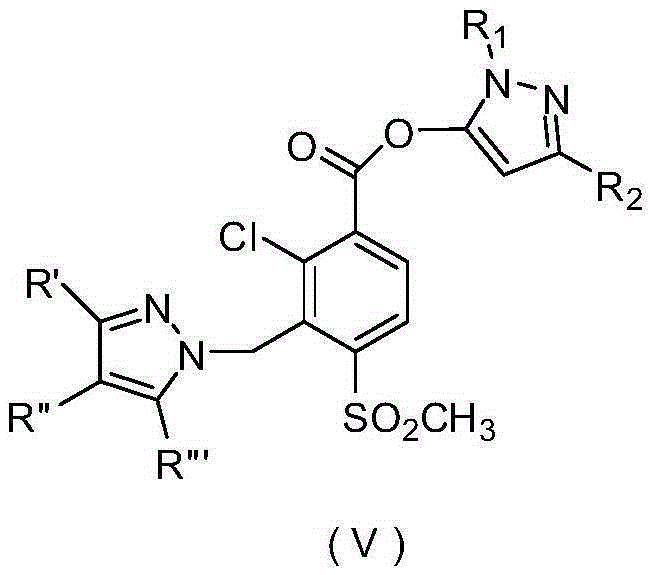
Pyrazole compound or salt thereof, and preparation method, herbicide composition and application thereof
A compound and pyrazole technology, applied in the field of pesticides, can solve problems such as the serious problem of barnyardgrass resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] This example illustrates the specific synthesis method of compound 01 in Table 1.
[0113] Step1: Synthesis of intermediate (a)
[0114]
[0115]Measure 50 mL of acetonitrile into a 250 mL three-neck flask, place in an ice-water bath, and control the temperature between 5 and 10 °C. Weigh 3.0g (0.075mol) NaH and slowly add it into a three-necked flask, control the temperature within 10°C, then weigh 3g (0.036mol) 4-methylpyrazole and dissolve it in a small amount of acetonitrile, put it in the dropping funnel, and wait When the temperature of the system drops to about 0°C, dropwise addition begins. After the dropwise addition was complete, stirring was continued under ice-water bath conditions. After the temperature of the system is stable, weigh 10g (0.030mol) of 2-chloro-3-bromomethyl-4-thiamphenicol benzoic acid and slowly add it to the system in batches. The temperature is controlled within 10°C and stirred in an ice-water bath. HPLC followed the reaction unti...
Embodiment 5
[0125] This example illustrates the specific synthesis method of compound 05 in Table 1.
[0126] Step1: Synthesis of intermediate (d)
[0127]
[0128] Measure 50 mL of acetonitrile into a 250 mL three-neck flask, place in an ice-water bath, and control the temperature between 5 and 10 °C. Weigh 4.4g (0.11mol) of NaH and slowly add it into a three-necked flask, control the temperature within 10°C, then weigh 4.6g (0.045mol) of 4-chloropyrazole and dissolve it in a small amount of acetonitrile, put it in a dropping funnel, and wait until When the temperature of the system drops to about 0°C, dropwise addition begins. After the dropwise addition was complete, stirring was continued under ice-water bath conditions. After the temperature of the system is stable, weigh 10g (0.030mol) of 2-chloro-3-bromomethyl-4-thiamphenicol benzoic acid and slowly add it to the system in batches. The temperature is controlled within 10°C and stirred in an ice-water bath. HPLC followed the r...
Embodiment 6
[0136] Example 6 illustrates the synthesis method of compound 06 in Table 1, which is similar to Example 5 and will not be described in detail here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com