Synthesis method of 3,5,6-trichloropyridin-2-ol sodium by using 2,3,5,6-tetrachloropyridine as raw material
A technology of tetrachloropyridine and trichloropyridine, which is applied in the field of organic compound synthesis, can solve the problems of increased production cost input, high hazards, and complicated production, and achieve the effects of reducing water costs, cheap prices, and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of method of using 2,3,5,6-tetrachloropyridine as raw material to synthesize 3,5,6-trichloropyridin-2-alcohol sodium of the present invention comprises the following steps:
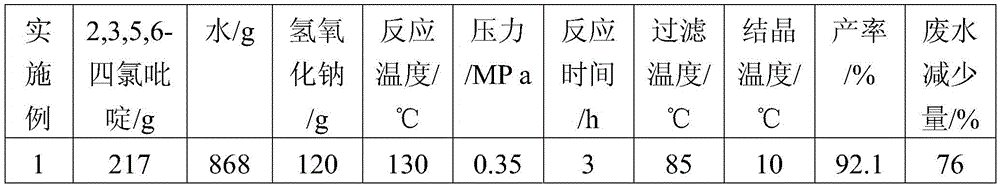
[0025] (1) Add 217g of 2,3,5,6-tetrachloropyridine to 868g of water, add 120g of sodium hydroxide, and react at 130°C and 0.35MPa for 3h to obtain 3,5,6-trichloropyridine Pyridin-2-alcohol sodium mixed solution, the temperature of the mixed solution is lowered to 85°C;
[0026] (2) Filter the mixed solution of 3,5,6-trichloropyridin-2-alcohol sodium in step (1) while it is hot, and remove a very small amount of unreacted 2,3,5,6-tetrachloropyridine solid , to obtain a filtrate, the 2,3,5,6-tetrachloropyridine solid obtained by filtering can be recovered as the reaction raw material of step (1) pressurized alkali analysis;
[0027] (3) Cool the primary filtrate of step (2) in an ice-water bath, cool down to 10°C, and precipitate sodium alkoxide crystals; then press filter to obtain white 3,5...
Embodiment 2
[0030] A kind of method of using 2,3,5,6-tetrachloropyridine as raw material to synthesize 3,5,6-trichloropyridin-2-alcohol sodium of the present invention comprises the following steps:
[0031] (1) Add 217g of 2,3,5,6-tetrachloropyridine to 868g of water, add 120g of sodium hydroxide, and react at 140°C and 0.4MPa for 4h to obtain 3,5,6-trichloropyridine Pyridin-2-alcohol sodium mixed solution, the mixed solution is cooled to 90°C;
[0032] (2) Filter the mixed solution of 3,5,6-trichloropyridin-2-alcohol sodium in step (1) while it is hot, and remove a very small amount of unreacted 2,3,5,6-tetrachloropyridine solid , to obtain a filtrate, the 2,3,5,6-tetrachloropyridine solid obtained by filtering can be recovered as the reaction raw material of step (1) pressurized alkali analysis;
[0033] (3) Cool the primary filtrate of step (2) in an ice-water bath, lower the temperature to 5°C, and precipitate sodium alkoxide crystals; then perform pressure filtration to obtain whit...
Embodiment 3
[0036] A kind of method of using 2,3,5,6-tetrachloropyridine as raw material to synthesize 3,5,6-trichloropyridin-2-alcohol sodium of the present invention comprises the following steps:
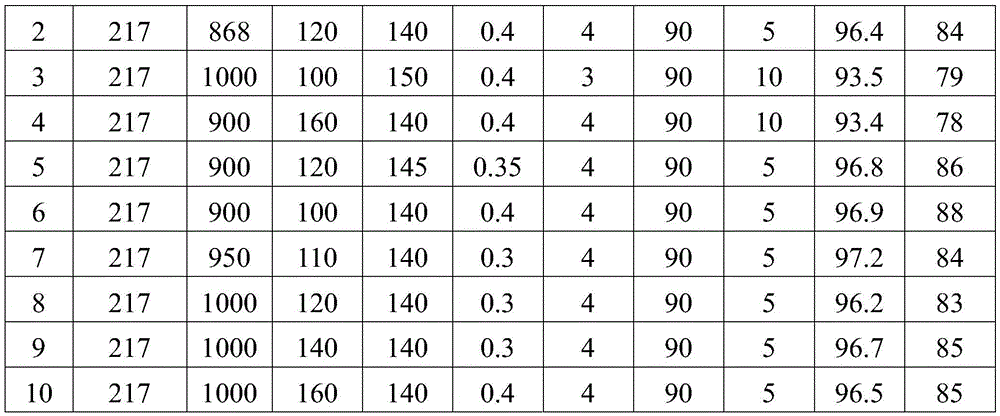
[0037] (1) Add 217g of 2,3,5,6-tetrachloropyridine to 1000g of water, add 100g of sodium hydroxide, and react at 150°C and 0.4MPa for 3h to obtain 3,5,6-trichloropyridine Pyridin-2-alcohol sodium mixed solution, the mixed solution is cooled to 90°C;
[0038] (2) Filter the mixed solution of 3,5,6-trichloropyridin-2-alcohol sodium in step (1) while it is hot, and remove a very small amount of unreacted 2,3,5,6-tetrachloropyridine solid , to obtain a filtrate, the 2,3,5,6-tetrachloropyridine solid obtained by filtering can be recovered as the reaction raw material of step (1) pressurized alkali analysis;
[0039] (3) Cool the primary filtrate of step (2) in an ice-water bath, cool down to 10°C, and precipitate sodium alkoxide crystals; then press filter to obtain white 3,5,6-trichloropyridin-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com