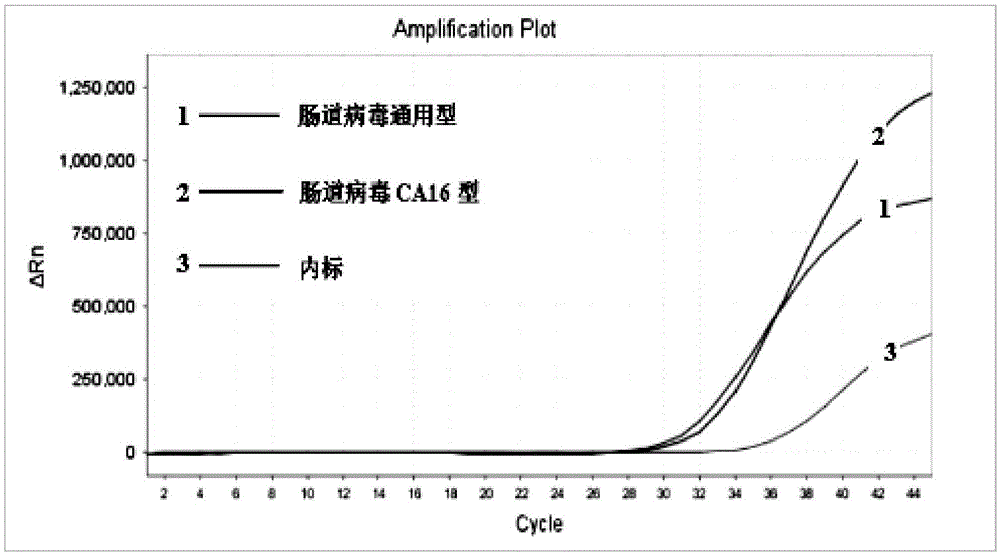
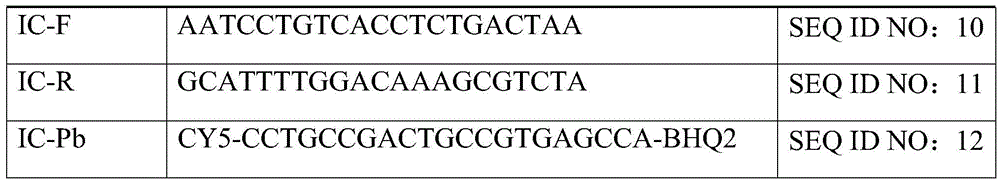
Enterovirus triplex direct fluorescence RT-PCR (reverse transcription-polymerase chain reaction) detection kit and reaction system
An RT-PCR, enterovirus technology, applied in the field of molecular biology detection of enterovirus, can solve the problems of mutual interference between primers and probes, lack of RT-PCR, etc., to shorten detection time, improve detection efficiency, reduce The effect of the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Kit of the present invention is used for the detection test of clinical sample
[0045] 1. Sample processing:
[0046] Pharyngeal swab samples: Use a sterile cotton swab to wipe the secretions from the palatine arches on both sides of the patient's mouth and pharynx and tonsils, avoiding touching other parts. Then quickly put the cotton swab into the collection tube provided by this kit, break the cotton swab rod near the top, add the internal standard solution, tighten the cap of the tube, mix well, and store it at 4°C for a short period of time.
[0047] 2. Reaction solution preparation:
[0048] In this embodiment, the concentration of Tris-HCl in the direct RT-PCR reaction solution is 31.25mmol / L, the concentration of ammonium sulfate in the direct RT-PCR reaction solution is 20.83mmol / L, and the concentration of potassium chloride in the direct RT-PCR reaction solution The concentration in the reaction solution is 72.92mmol / L, and the volume percenta...
Embodiment 2
[0060] Example 2 Comparison of analytical sensitivity between triple direct fluorescent RT-PCR and single conventional reagents for hand, foot and mouth
[0061] The nucleic acid sample of the CA16 standard strain of enterovirus was diluted to a nucleic acid concentration of 0.001LD50; according to the method and steps in Example 1, triple direct fluorescent RT-PCR was compared with single-fold routine reagent amplification detection, each concentration Repeat the detection 30 times. The results are shown in Table 3.
[0062] Table 3 Analytical Sensitivity Results
[0063]
[0064]
[0065] From the results shown in table 3, the analytical sensitivity of the direct fluorescent RT-PCR method of the present invention detects enterovirus is compared with the detection sensitivity of conventional reagent single-fold RT-PCR with purified RNA as a template, and this result illustrates that the direct fluorescent RT-PCR method The analytical sensitivity for detection of enter...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap