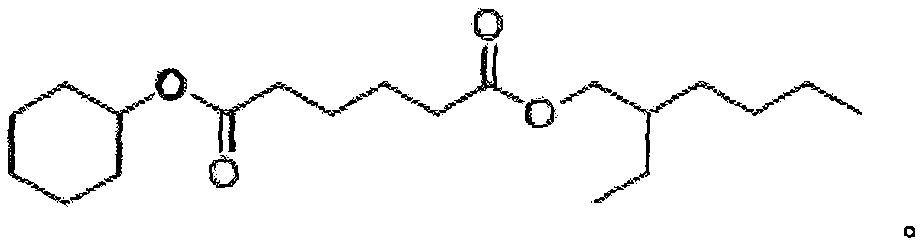
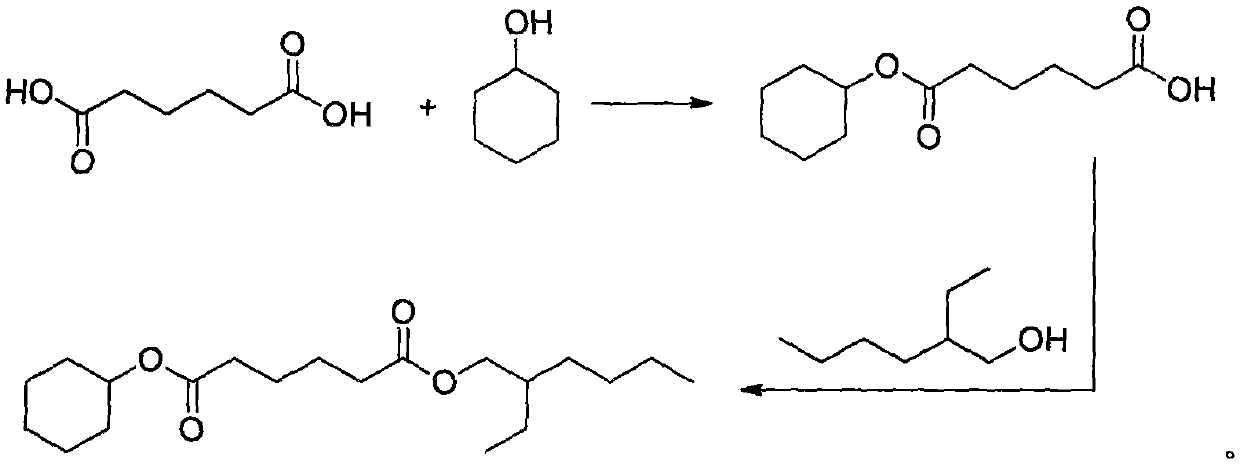
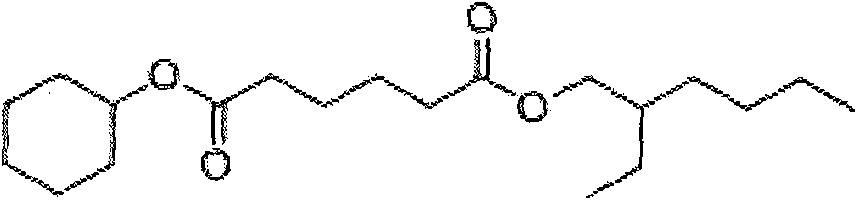
Asymmetric adipic acid cyclohexanol isooctyl alcohol ester and synthetic method and application thereof
A synthesis method and isooctanol ester technology are applied in the field of asymmetric cyclohexanol isooctyl adipate and its synthesis, which can solve the problems of limited application scope, complicated production process, poor comprehensive performance and the like, and achieve a product High yield, good softness, good cold resistance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthetic method of asymmetric cyclohexanol isooctyl adipate, its synthetic concrete steps are: a, adipic acid (146.14g, 1.0mol), cyclohexanol (100.06g, 1.0mol) , 98% concentrated sulfuric acid (1.00g, 0.01mol) and toluene (250mL) were added in a 1L three-necked flask, and heated to reflux in an oil bath at 100°C to separate water until anhydrous was formed; b. Add isooctyl alcohol to the reactor (130.23g, 1.0mol), continue to heat at 100°C in an oil bath to reflux to separate water until anhydrous is formed, and cool to room temperature; c, rotary evaporation at 45°C to remove toluene; d, vacuum distillation at 180°C to obtain asymmetric hexadiene Acid cyclohexyl isooctyl ester (82% yield, 279.2 g), colorless liquid.
Embodiment 2
[0022] Embodiment 2: the synthetic method of asymmetric cyclohexanol isooctyl adipate, its synthetic concrete steps are: a, adipic acid (146.14g, 1.0mol), cyclohexanol (130.08g, 1.3mol) , Concentrated hydrochloric acid (1.82g, 0.05mol) and cyclohexane (300mL) were added to a 1L three-necked flask, and heated to reflux in an oil bath at 105°C to separate water until anhydrous was formed; b. Add isooctyl alcohol to the reactor (195.35g, 1.5mol), continue to heat at 105°C in an oil bath to reflux to separate water until anhydrous is formed, and cool to room temperature; c, rotary evaporation at 40°C to remove cyclohexane; d, vacuum distillation at 180°C to obtain asymmetric Cyclohexyl isooctyl adipate (78% yield, 265.59 g), colorless liquid.
Embodiment 3
[0023] Embodiment 3: the synthetic method of asymmetric cyclohexanol isooctyl adipate, its synthetic concrete steps are: a, adipic acid (146.14g, 1.0mol), cyclohexanol (120.19g, 1.2mol) , 98% concentrated sulfuric acid (1.50g, 0.015mol) and toluene (300mL) were added in a 1L three-necked flask, and heated to reflux in an oil bath at 110°C to separate water until anhydrous was generated; b. Add isooctyl alcohol to the reactor (169.30g, 1.3mol), continue to heat at 110°C in an oil bath to reflux to separate water until anhydrous is formed, and cool to room temperature; c, rotary evaporation at 45°C to remove toluene; d, vacuum distillation at 180°C to obtain asymmetric hexadiene Acid cyclohexyl isooctyl ester (97% yield, 330.29 g), colorless liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com