Human primary cell culture medium and application thereof
A primary cell and culture medium technology, applied in tissue culture, culture, microorganisms, etc., can solve problems such as troublesome operation steps, insufficient practicability, and differences in primary cell culture effects, and achieve high amplification efficiency and long culture time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 evaluates the success rate of culturing human primary B-ALL cells in vitro using the culture medium of the present invention
[0047] Human primary B-ALL cells were isolated and obtained by density centrifugation, and the specific steps were as follows:
[0048] 1) Mix the bone marrow or peripheral blood samples of B-ALL patients with normal saline in equal proportions;
[0049] 2) Into a new 15mL centrifuge tube, add one-half of the volume of diluted blood lymphatic separation solution (Lymphoprep, StemCellTechnologies);
[0050] 3) Slowly superimpose the diluted blood on the layered liquid surface along the wall of the centrifuge tube, keeping the liquid surface clear;
[0051] 4) Gently put the bone marrow sample mixture centrifuge tube into the centrifuge, and centrifuge at 800g / min for 20min;
[0052] 5) Gently take out the centrifuge tube, layer the bone marrow sample mixture, use a Pasteur pipette to carefully absorb the cells in the middle cloud la...
Embodiment 2
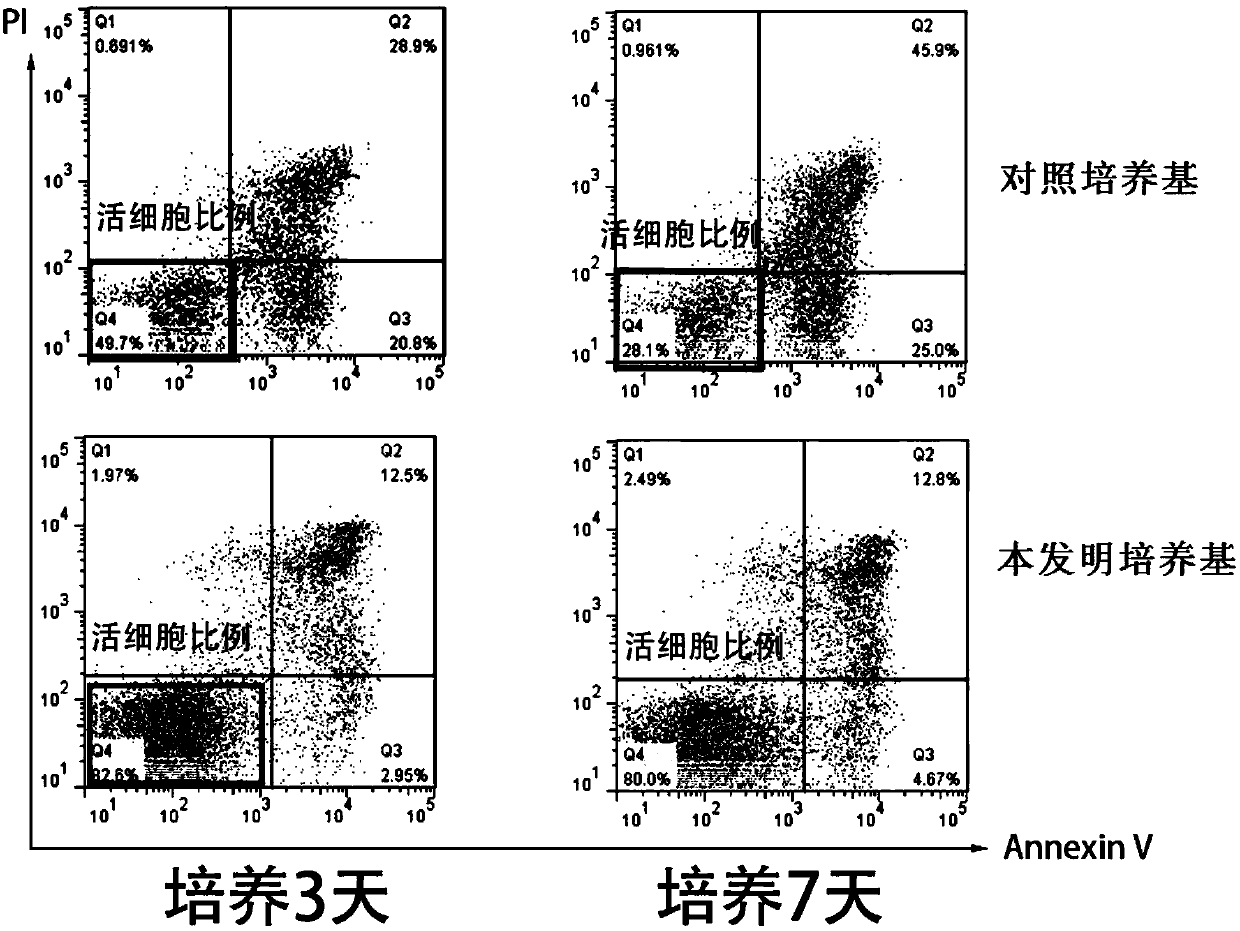
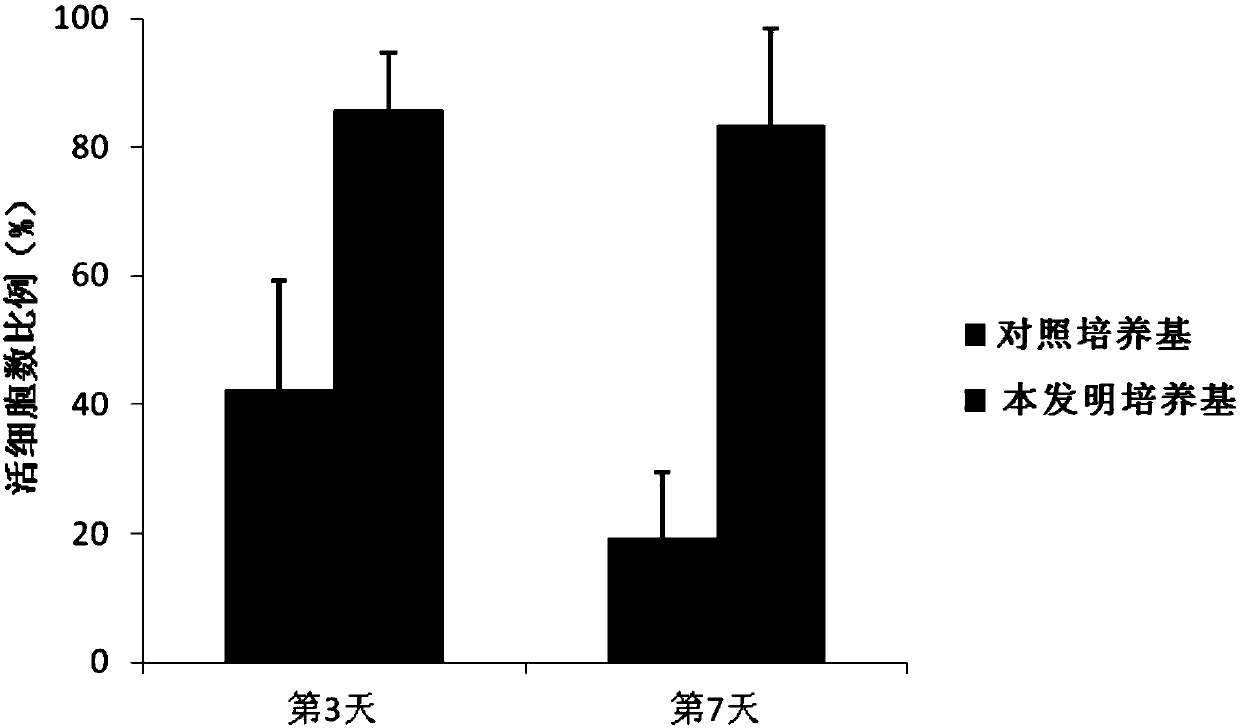
[0059] Example 2 evaluates the cell viability of culturing human primary B-ALL cells in vitro using the culture medium of the present invention
[0060] Using the human primary B-ALL cell sample prepared in Example 1, using the control medium containing serum but not containing cytokine combination as a control, evaluate the cell survival of human primary B-ALL cells cultured in vitro using the medium of the present invention Rate. The composition of the control medium is: IMDM+10%FBS+1% / P / S+5mM glutamate; the composition of the medium of the present invention is: IMDM+1% / P / S+5mM glutamate+cytokines combination , the composition and content of the cytokine combination are shown in the following table:
[0061] Element
concentration
BSA
1mg / ml
10ug / ml
10ug / ml
humanFLT3L
100ng / ml
[0062] hIGF1
100ng / ml
hIL-7
50ng / ml
hIL-6
50ng / ml
[0063] Specific...
Embodiment 3
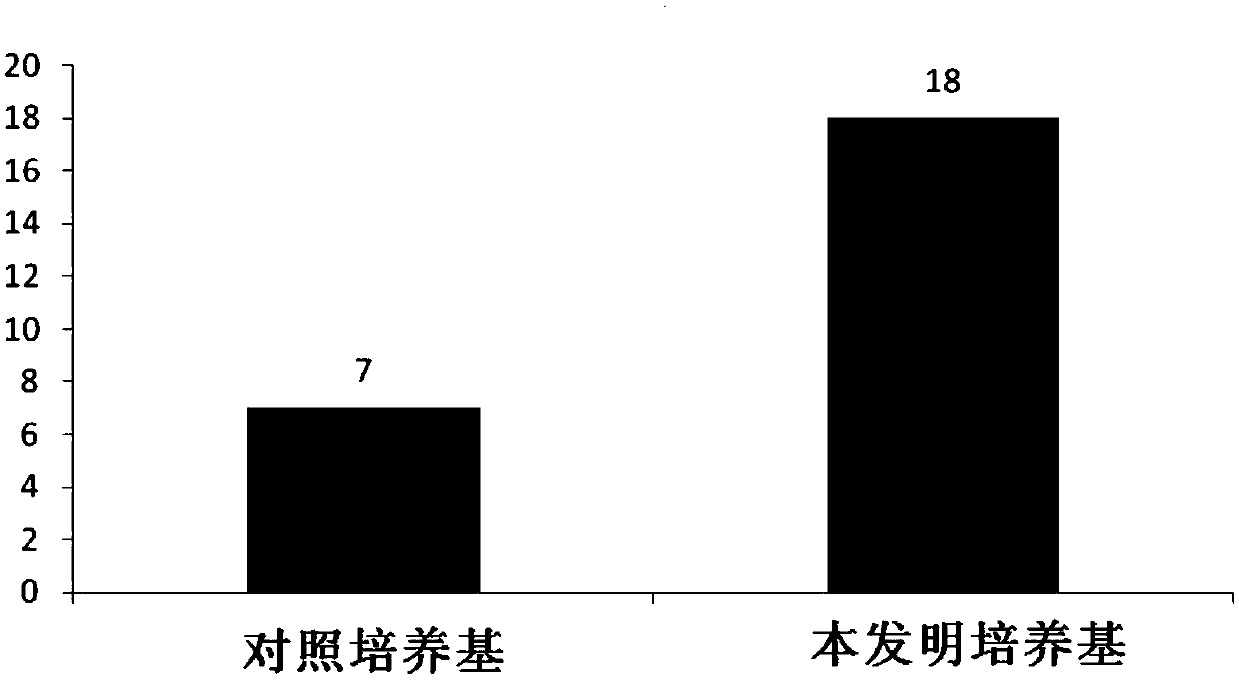
[0065] Example 3 Use the culture medium of the present invention to culture the cell growth curve of human primary B-ALL cells in vitro
[0066] Using the human primary B-ALL cell sample prepared in Example 1, using the control medium containing serum but not containing cytokine combination as a control, evaluate the cell growth of human primary B-ALL cells cultured in vitro using the medium of the present invention situation. The composition of the control medium is: IMDM+10%FBS+1% / P / S+5mM glutamate; the composition of the medium of the present invention is: IMDM+1% / P / S+5mM glutamate+cytokines combination , the composition and content of the cytokine combination are shown in the following table:
[0067] Element
concentration
BSA
5mg / ml
5
5ug / ml
5ug / ml
humanFLT3L
50ng / ml
hIGF1
50ng / ml
hIL-7
20ng / ml
hIL-6
20ng / ml
[0068] Specifically, the 5×10 5 The primary B-ALL cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com