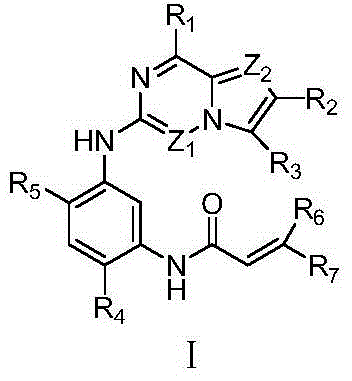
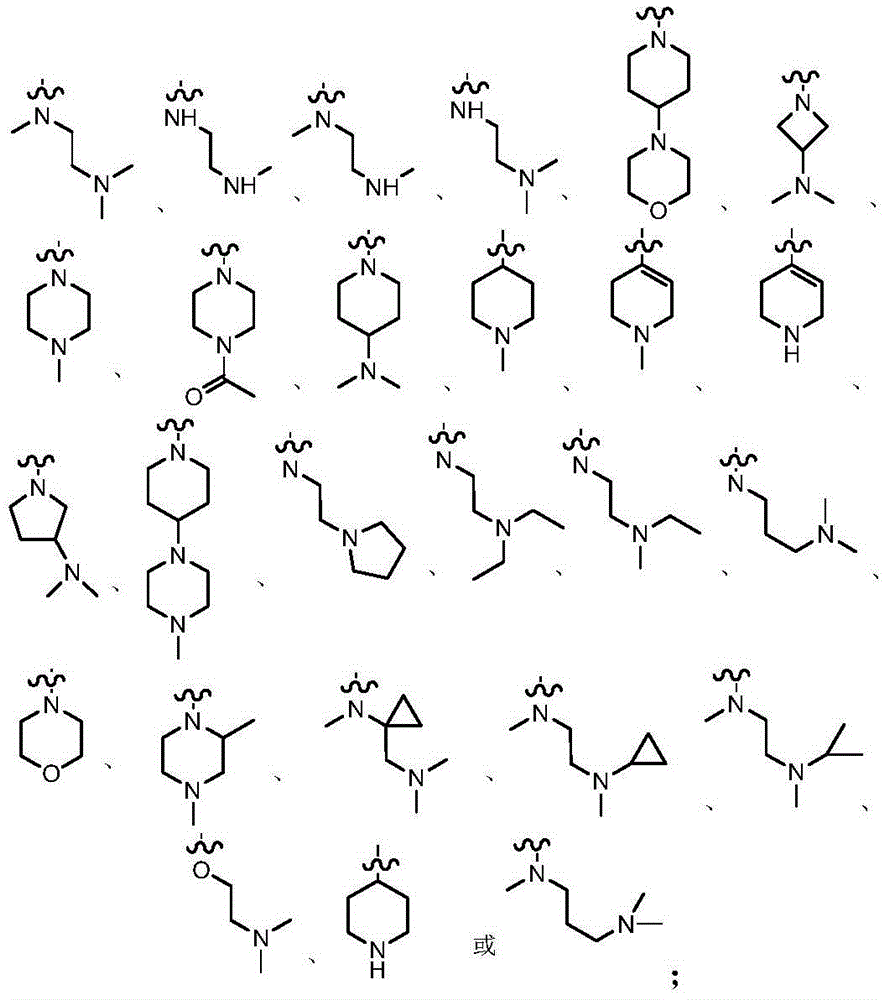
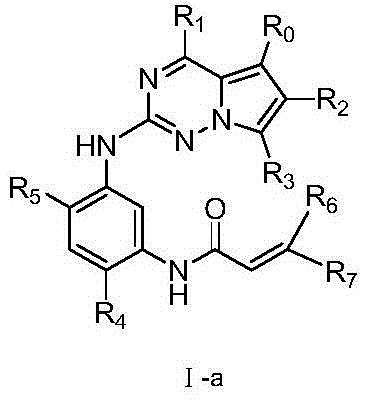
Azabicyclo derivatives, preparation methods thereof, and pharmaceutical applications thereof
一种药学、前药的技术,应用在医药领域,能够解决耐药病人疗效差、病人皮疹毒副作用严重等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0170] Preparation of compound 1-a
[0171]
[0172]
[0173] Step a: In a 4L flask, 25.0g of raw material 1-a-1 (0.19mol) was dissolved in 1L of tetrahydrofuran, stirred at room temperature under nitrogen protection for 20 minutes, and 1M potassium tert-butoxide in tetrahydrofuran (500.0ml, 0.50mol ) was added to the solution and stirred for 1 hour. Then 0.15M chloramine ether solution (2.1L, 0.31mol) was added to the reaction solution within 20 minutes at 10°C, and nitrogen gas was introduced at the same time. After 2 hours, saturated aqueous sodium thiosulfate solution (500ml) was added gradually The reaction solution was added dropwise and stirring was continued for one hour. The organic phase was separated, washed with water and saturated brine, and dried over anhydrous sodium sulfate. After filtering off the desiccant, the filtrate was concentrated under reduced pressure to obtain compound 1-a-2 as an oil. MSm / z(ESI):141.2[M+H] + .
[0174] Step b: Dissolve th...
Embodiment 1
[0196] Example 1: N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(7-(1-methyl-1H-pyrazole Preparation of [1,2,4]triazin-2-ylamino)phenyl)acrylamide (J-1) of -4-yl)pyrrolo[1,2-f]
[0197]
[0198]
[0199] Step 1: Compound 1-a (6.0 g, 24.6 mmol), 1-methyl-4(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) -1H-pyrazole (10.2g, 49.0mmol), tetrakistriphenylphosphopalladium (2.84g, 2.46mmol), potassium carbonate (10.2g, 73.8mmol), dioxane 60ml, water 20ml mixture, argon Protected, stirred at 100°C for 4 hours. After the reaction was completed, cool to room temperature, add dichloromethane and water, separate the organic phase, and concentrate under reduced pressure to obtain the crude product, which was purified by Combi-flash column chromatography [PE:EA=100:0~0:100] to obtain compound 1 as a yellow solid -b (5.7g), directly used in the next reaction. Yield: 94.5%; Purity: 98.76% (UV254). MSm / z(ESI):246.0[M+H] + .
[0200] Step 2: Compound 1-b (5.64g, 22.7mmol) w...
Embodiment 2
[0204] Example 2: N-(2-(4-(dimethylamino)piperidin-1-yl)-4-methoxy-5-(7-(1-methyl-1H-pyrazole-4- Preparation of [11,2,4]triazin-2-ylamino)phenyl)acrylamide (J-2) of pyrrolo[1,2-f]
[0205]
[0206] Step: Add compound 1-e (50 mg, 0.098 mmol), compound 3-a (32 mg, 0.1 mmol), tris(dibenzylideneacetone) dipalladium (9 mg, 0.0098 mmol) to 4 ml of dioxane solution, 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (9mg, 0.016mmol), cesium carbonate (64mg, 0.196mmol), under argon protection, stirred at 120°C for 10 minutes under microwave. After the reaction was completed, cool to room temperature, filter with diatomaceous earth, wash the filter cake with dichloromethane, and concentrate the filtrate under reduced pressure to obtain a crude product, which was separated and purified by preparative liquid phase to obtain compound J-2 (5.67 mg) as a yellow solid, with a yield of 11.2%; Purity: 100% (UV254). MSm / z(ESI):516.0[M+H] + . 1 HNMR(400MHz,DMSO)δ9.11(s,1H),8.84(s,1H),8.48(s,1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com