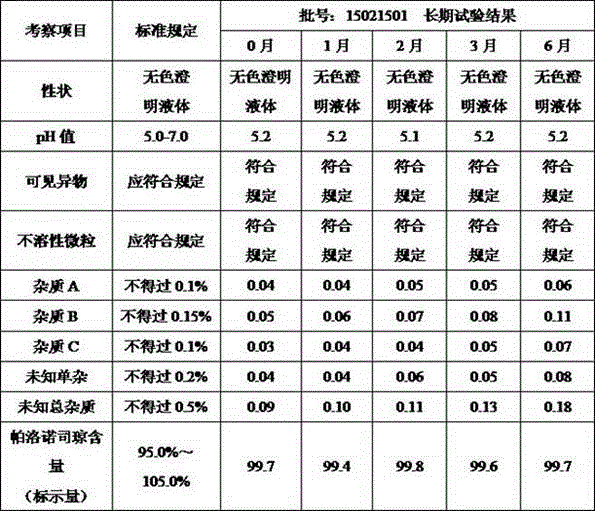
Palonosetron hydrochloride injection and preparation method thereof
A technology for palonosetron and chon injections, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, drug delivery and other directions, can solve problems such as increasing the pain of patients, achieve enhanced medication compliance, reduce metal ion precipitation, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
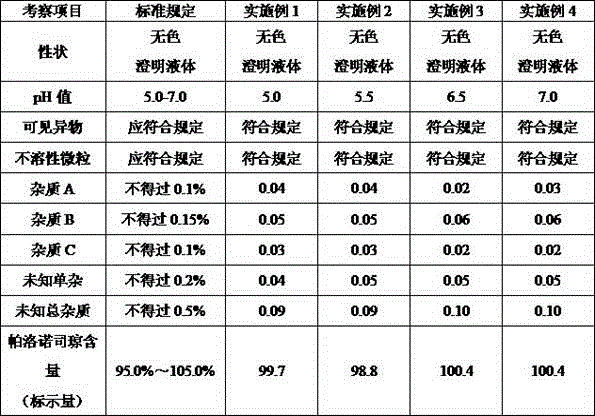
Embodiment 1
[0024] prescription:
[0025] Palonosetron hydrochloride (calculated as palonosetron) 0.075mg (equivalent concentration 0.0015mg / ml)
[0027] Add water for injection to 50ml
[0028] Preparation:
[0029] (1) Put the prescribed amount of palonosetron hydrochloride and sodium chloride into the water for injection that has been filled with nitrogen and deoxygenated, stir and dissolve, and the measured pH value should be 5.0;
[0030] (2) Under the protection of continuous nitrogen filling, filter through 0.45μm and 0.22μm filter membranes, fill and seal with plastic packaging;
[0031] (3) Sterilize in a high-pressure water bath at 121°C for 12-15 minutes.
Embodiment 2
[0033] prescription:
[0034] Palonosetron hydrochloride (calculated as palonosetron) 0.075mg (equivalent to 0.00075mg / ml)
[0036] Add water for injection to 100ml
[0037] Preparation:
[0038] (1) Put the prescribed amount of palonosetron hydrochloride and sodium chloride into the water for injection that has been filled with nitrogen and deoxygenated, stir and dissolve, and the measured pH value should be 5.5;
[0039] (2) Under the protection of continuous nitrogen filling, filter through 0.45μm and 0.22μm filter membranes, fill and seal with plastic packaging;
[0040] (3) Sterilize in a high-pressure water bath at 121°C for 12-15 minutes.
Embodiment 3
[0042] prescription:
[0043] Palonosetron hydrochloride (calculated as palonosetron) 0.25mg (equivalent concentration 0.005mg / ml)
[0044] Sodium chloride 0.45g
[0045] Add water for injection to 50ml
[0046] Preparation:
[0047] (1) Put the prescribed amount of palonosetron hydrochloride and sodium chloride into the water for injection that has been filled with nitrogen and deoxygenated, stir and dissolve, and the measured pH value should be 6.5;
[0048] (2) Under the protection of continuous nitrogen filling, filter through 0.45μm and 0.22μm filter membranes, fill and seal with plastic packaging;
[0049] (3) Sterilize in a high-pressure water bath at 121°C for 12-15 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com