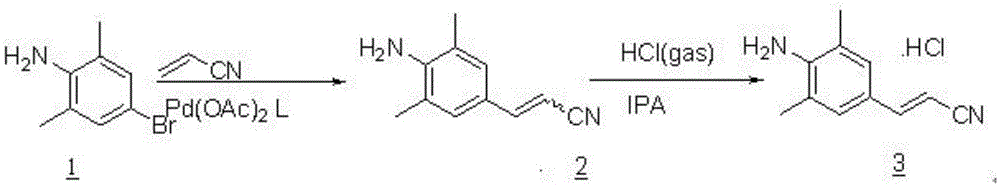
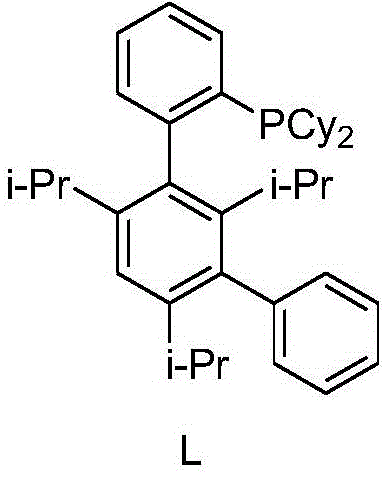
Rilpivirine midbody preparing technology
A preparation technology, the technology of rilpivirine, is applied in the field of preparation technology for the synthesis of rilpivirine intermediates, which can solve the problems of high catalyst cost, harsh process conditions, troublesome post-processing, etc., and achieve good product quality, simple operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (compound 2 Synthesis)
[0016] A 20L four-necked flask containing a thermometer, mechanical stirring, a constant pressure dropping funnel and a tail gas absorption device was replaced with nitrogen three times, and THF (10L), palladium acetate (5.6g, 0.5% mol) and phosphine ligand L (28g, were added) were added. 1% mol), stirred at 20-30 °C for 1 hour. Sodium acetate (1.02Kg, 7.5mol), 4-bromo-2,6-dimethylaniline (1.0Kg, 5mol) and acrylonitrile (405g, 7.5mol) were added sequentially. The reaction was continued to stir at 20-30°C for 4 hours, then water (5.0L) was added, and the layers were allowed to stand for separation. The organic phase was separated, and the aqueous phase was extracted once with methyl tertiary ether (5L). The organic phases were combined and concentrated under reduced pressure to obtain 870 g of crude 3-(4-amino-3,5-dimethylphenyl)acrylonitrile (trans:cis=4.6:1), with a crude yield of 102%.
[0017] (compound 3 Synthesis)
[0018] In a 20L fou...
Embodiment 2
[0022] (compound 2 Synthesis)
[0023] A 20L four-necked flask containing a thermometer, mechanical stirring, a constant pressure dropping funnel and a tail gas absorption device was replaced with nitrogen three times, and THF (10L), palladium acetate (1.2g, 0.1% mol) and phosphine ligand L (6g, 0.2% mol), stirring at 20-30 °C for 1 hour. Sodium acetate (1.02Kg, 7.5mol), 4-bromo-2,6-dimethylaniline (1.0Kg, 5mol) and acrylonitrile (405g, 7.5mol) were added sequentially. The reaction was continued to stir at 20-30°C for 4 hours, then water (5.0L) was added, and the layers were allowed to stand for separation. The organic phase was separated, and the aqueous phase was extracted once with methyl tertiary ether (5L). The organic phases were combined and concentrated under reduced pressure to obtain 870 g of crude 3-(4-amino-3,5-dimethylphenyl)acrylonitrile (trans:cis=4.7:1), with a crude yield of 102%.
[0024] (compound 3 Synthesis)
[0025] In a 20L four-necked flask equippe...
Embodiment 3
[0027] (compound 2 Synthesis)
[0028] A 20L four-necked flask containing a thermometer, mechanical stirring, a constant pressure dropping funnel and a tail gas absorption device was replaced with nitrogen three times, and THF (10L), palladium acetate (5.6g, 0.5% mol) and phosphine ligand L (28g, were added) were added. 1% mol), stirred at 20-30 °C for 1 hour. Sodium acetate (1.02Kg, 7.5mol), 4-bromo-2,6-dimethylaniline (1.0Kg, 5mol) and acrylonitrile (405g, 7.5mol) were added sequentially. The reaction was continued to stir at 20-30°C for 4 hours, then water (5.0L) was added, and the layers were allowed to stand for separation. The organic phase was separated, and the aqueous phase was extracted once with methyl tertiary ether (5L). The organic phases were combined and concentrated under reduced pressure to obtain 870 g of crude 3-(4-amino-3,5-dimethylphenyl)acrylonitrile (trans:cis=4.6:1), with a crude yield of 102%.
[0029] (compound 3 Synthesis)
[0030] In a 20L fou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com