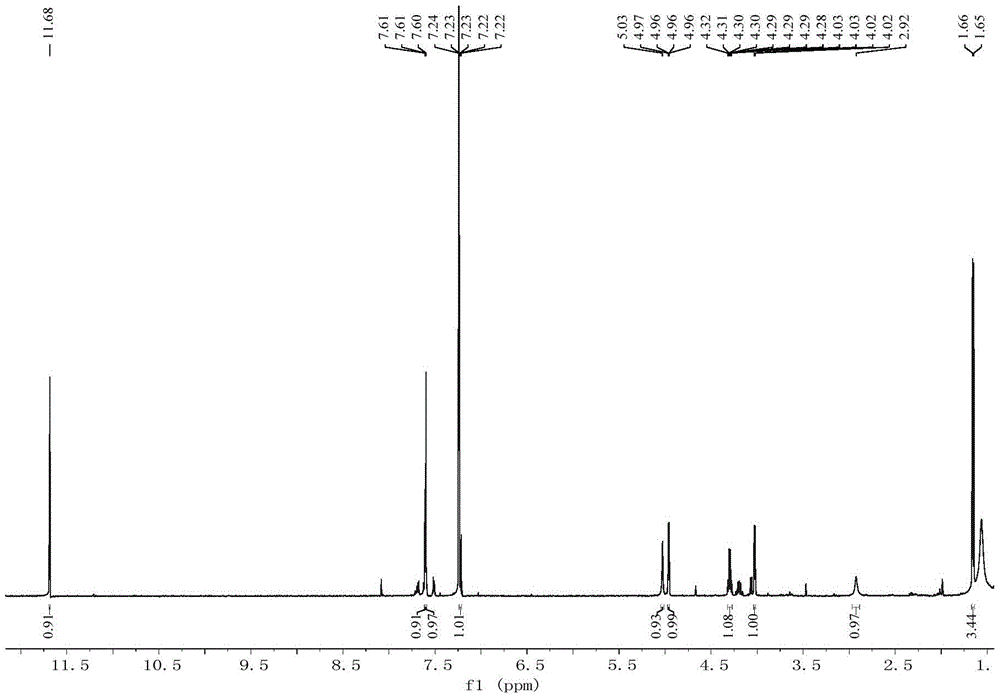
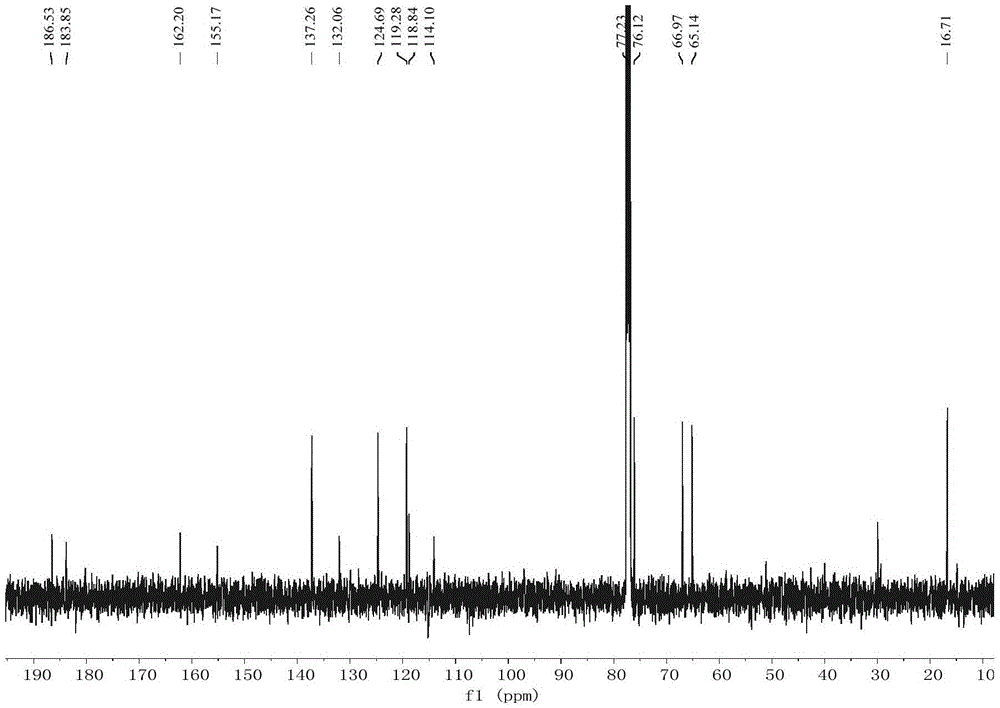
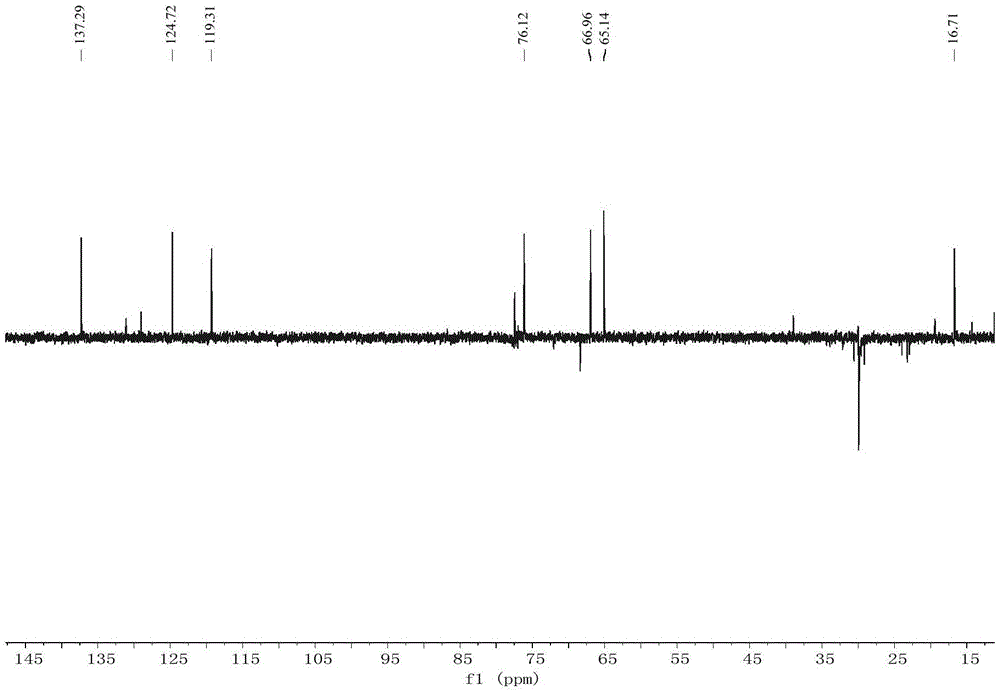
Compound acaromycin A and preparation method thereof and application of compound in preparation of antitumor drug
A technology of anti-tumor drugs and compounds, applied in the field of biology and medicine, can solve the problems of secondary metabolite research without Acaromyces fungi, and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The deep-sea fungus Acaromycesingoldii FS121 of activation is inserted into the potato dextrose liquid culture medium containing mass fraction 1.5% sea salt (every liter contains 15g sea salt, 200g potato, 20g of glucose, the remainder is water, and its preparation method is: the potato is washed and peeled, Cut to about 1cm in size 3 Weigh 200g of small pieces, put them in 1L of water and boil for 20-30 minutes, filter with double gauze, take the filtrate, add 15g of sea salt and 20g of glucose to the filtrate, add water to make up 1L, and sterilize for later use. ), 28°C, 120rpm, cultured for 5 days to obtain seed solution, and the seed solution was inserted into a new potato glucose liquid medium containing 1.5% sea salt in mass fraction with an inoculum size of 10% by volume, at 28°C, under 120rpm Shaking culture was carried out for 7 days to obtain a fermentation culture.
[0033] The fermentation broth and mycelia of the fermentation culture are separated, the fe...
Embodiment 2
[0041] The growth inhibitory activity of compound acaromycin A on tumor cells was tested by SRB method (SkehanP. et al. 1990).
[0042] The tumor cell lines are glioma cell SF-268, breast cancer cell MCF-7, non-small cell lung cancer cell NCI-H460 and liver cancer cell HePG-2.
[0043] 1. Experimental method: the compound acaromycinA prepared by the present invention is dissolved with dimethyl sulfoxide (DMSO) to obtain a mother solution with a concentration of 10mmol / L, and then diluted to the required concentration with RPMI-1640 medium, and the positive control drug is cisplatin . Take the NCI-H460, SF-268, MCF-7 and HePG-2 cells in the logarithmic growth phase, digest them with trypsin, and count them with trypan blue staining. After the trypan blue exclusion test shows that the cell viability is greater than 95%, use fresh RPMI -1640 medium to adjust the cell concentration to 3×10 4 cells / mL, the cells were seeded in a 96-well plate, 180 μL of cell suspension was added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com