Crystal forms A, H and I of barrack gefitinib phosphate and preparation methods thereof
A technology of phosphate and crystal form of tinib, which is applied in the field of polymorphic forms of baricitinib derivatives, and can solve the problems of increasing solubility of phosphate and affecting bioavailability of baricitinib.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
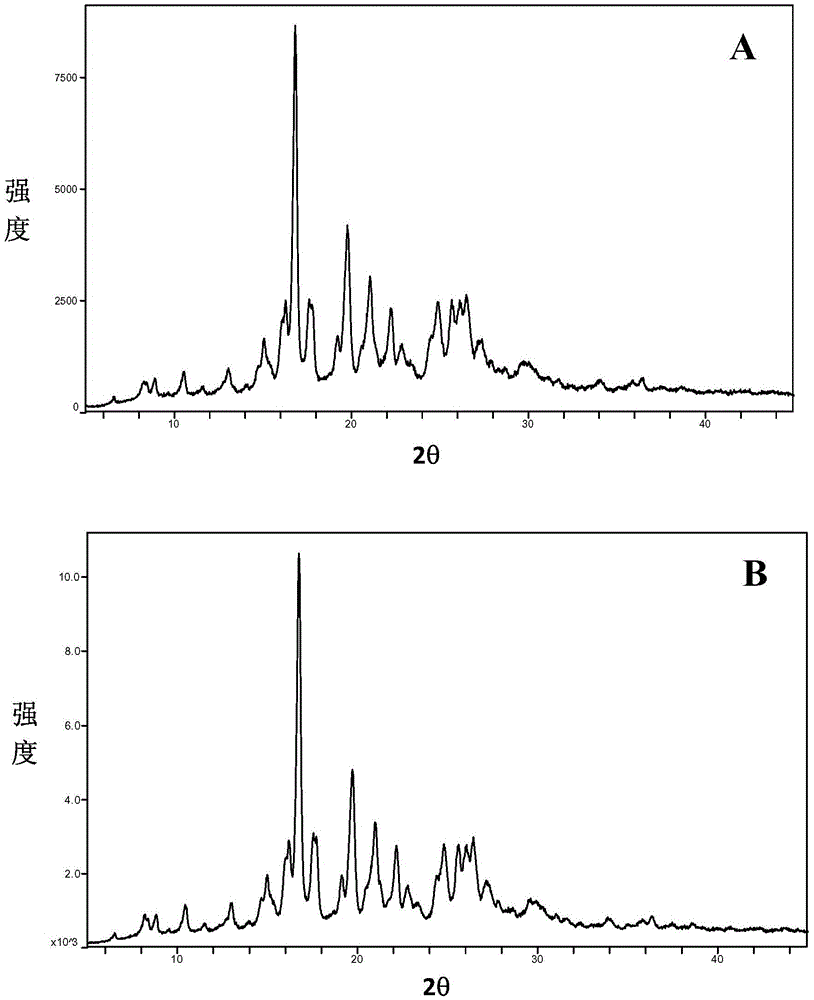
Image
Examples
Embodiment 1 to 5
[0093] Examples 1 to 5 Preparation of Baricitinib Phosphate A Crystal Form
[0094] Weigh 1.0 g of baricitinib phosphate into a sample bottle, add 5 mL of N,N dimethylformamide to completely dissolve it, and then slowly add the solvents in Table 1 to the dissolved product. After a solid precipitated from the mixture of the dissolved substance and the organic solvent, an additional 5 mL of the organic solvent was added dropwise. The resulting mixture was allowed to stand overnight at room temperature, and then the precipitate obtained after standing overnight was filtered and vacuum-dried to obtain an off-white solid, which was weighed to calculate its yield, and the results are shown in Table 1. The reagents used in the process were all analytically pure.
[0095] Table 1 Preparation of baricitinib A crystal form
[0096] Example
Embodiment 6
[0097] Example 6 Characterization of baricitinib phosphate A crystal form by XRPD pattern
[0098] The measurement of the X-ray powder diffraction (XRPD) pattern is carried out using the RigakuUltimaIV model combined multifunctional X-ray diffractometer, and the specific collection information is as follows: Cu anode (40kV, 40mA), scanning speed 20° / min, scanning range (2θ range) 3~45°, scan step size 0.02, slit width 0.01. Samples were processed using glass slides pressed directly onto the test plate. Subsequent XRPD patterns all adopt similar measurement methods.
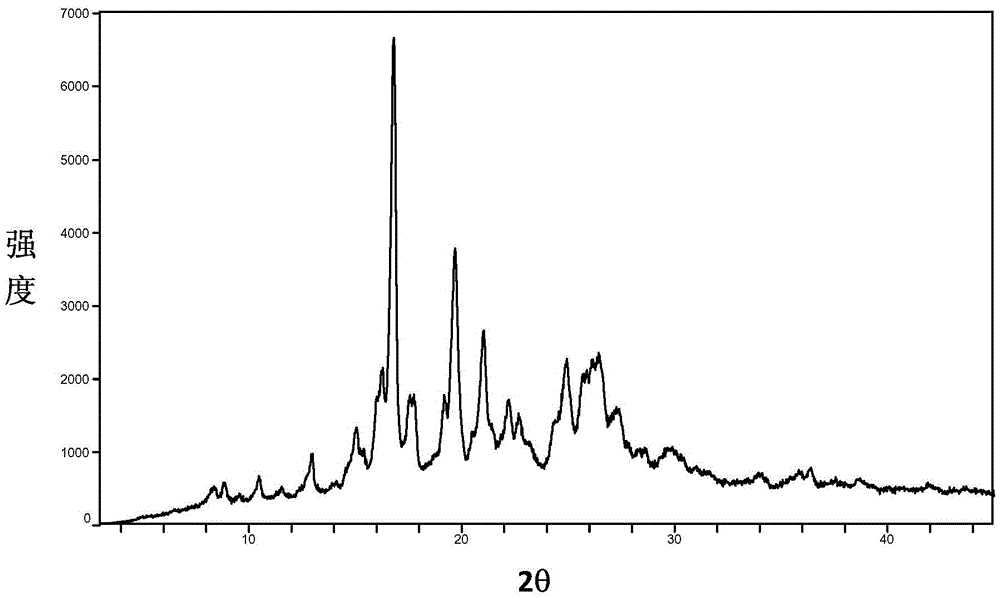
[0099] Determination of the XRPD pattern of the baricitinib phosphate A crystal form prepared according to the method described in Example 1, at 2θ=8.38, 8.88, 10.48, 13.00, 15.06, 16.22, 16.82, 17.76, 19.18, 19.70, 21.04, 22.22 , There are diffraction peaks at 22.68, 24.40, 24.94, 26.48, and 27.40, such as figure 1 shown. The error range of 2θ value is ±0.2. After testing, the error range of 2θ value can als...
Embodiment 7
[0101] Example 7 Investigation of the High Temperature Stability of Baricitinib Phosphate A Crystal Form
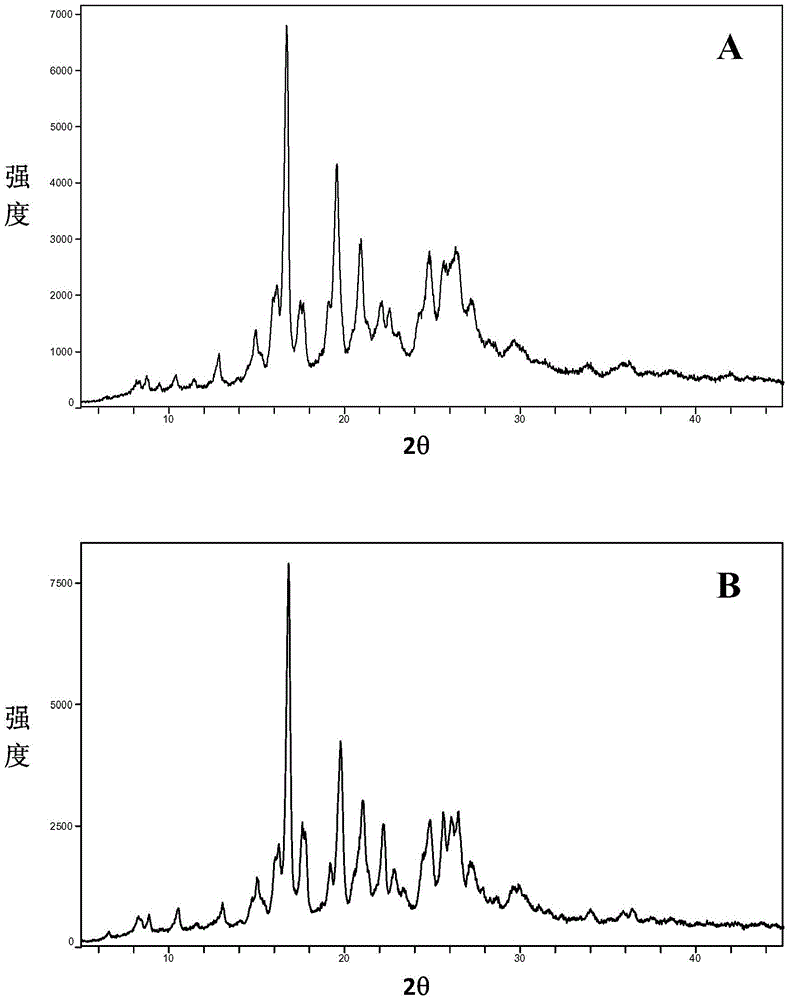
[0102] Take an appropriate amount of baricitinib phosphate A crystal form and place it in a 60°C oven, take out the sample after 5 days and 10 days for XRPD testing (such as figure 2 Shown), to investigate the crystal form stability of baricitinib phosphate A crystal form to temperature. The results showed that the crystalline form of baricitinib phosphate A was stable under high temperature conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com