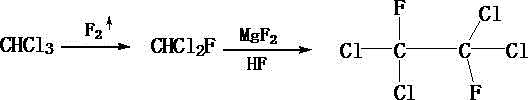Synthesis method of polydifluorodichloroethylene
A polydifluoroethylene, synthetic method technology, applied in the field of synthesis, can solve the problems of poor mechanical properties and resilience properties, difficulties in forming and secondary processing, large linear expansion coefficient, etc., and achieve mechanical properties and resilience properties Good, small linear expansion coefficient, and improved strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
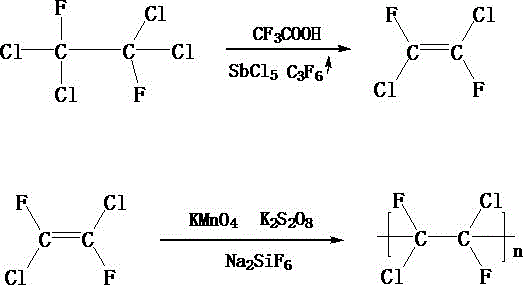
[0018] First measure 100mL of chloroform, put it into a sealed container, feed fluorine gas from the bottom of the sealed container, control the rate of fluorine gas introduction to 23mL / s, and stop feeding fluorine gas when the fluorine gas content in the sealed container is 78%. , control the temperature in the airtight container to 62°C, fluorinate for 2 hours to obtain chlorofluoromethane; then add 3g of magnesium fluoride to the fluorodichloromethane obtained above, and stir at 80r / min for 20min while stirring Add 4mL of anhydrous hydrofluoric acid dropwise to it at a rate of 2 drops / s. After the dropwise addition, react at a temperature of 50°C for 3h to obtain difluorodichloroethane; Put ethane into the reaction kettle, add 20mL trifluoroacetic acid and 15mL antimony pentachloride to the reaction kettle at the same time, stir to make it fully mixed, and then feed hexafluoropropylene gas into it, and control the feeding rate to 20mL / s, when the hexafluoropropylene gas c...
example 2
[0020] First measure 110mL of chloroform, put it into a sealed container, feed fluorine gas from the bottom of the sealed container, control the rate of fluorine gas introduction to 24mL / s, and stop feeding fluorine gas when the fluorine gas content in the sealed container is 80%. , control the temperature in the sealed container at 64°C, and perform fluorination reaction for 3 hours to obtain chlorofluoromethane; then add 4 g of magnesium fluoride to the chlorofluoromethane obtained above, and stir at 80 r / min for 25 minutes while stirring Add 5 mL of anhydrous hydrofluoric acid dropwise to it at a rate of 3 drops / s. After the dropwise addition, react at a temperature of 55°C for 4 hours to obtain difluorodichloroethane; Put ethane into the reaction kettle, add 23mL trifluoroacetic acid and 18mL antimony pentachloride to the reaction kettle at the same time, stir to make it fully mixed, and then feed hexafluoropropylene gas into it, and control the feeding rate to 21mL / s, wh...
example 3
[0022] First measure 120mL of chloroform, put it into a sealed container, feed fluorine gas from the bottom of the sealed container, control the rate of fluorine gas introduction to 25mL / s, and stop feeding fluorine gas when the fluorine gas content in the sealed container is 82%. , control the temperature in the sealed container to 66°C, and perform fluorination reaction for 3 hours to obtain chlorofluoromethane; then add 5 g of magnesium fluoride to the chlorofluoromethane obtained above, and stir at 80 r / min for 30 min while stirring Add 6mL of anhydrous hydrofluoric acid dropwise to it at a rate of 3 drops / s. After the dropwise addition, react at a temperature of 60°C for 4 hours to obtain difluorodichloroethane; Put ethane into the reaction kettle, add 25mL trifluoroacetic acid and 20mL antimony pentachloride to the reaction kettle at the same time, stir to make it fully mixed, and then feed hexafluoropropylene gas into it, and control the feeding rate to 23mL / s, when th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com