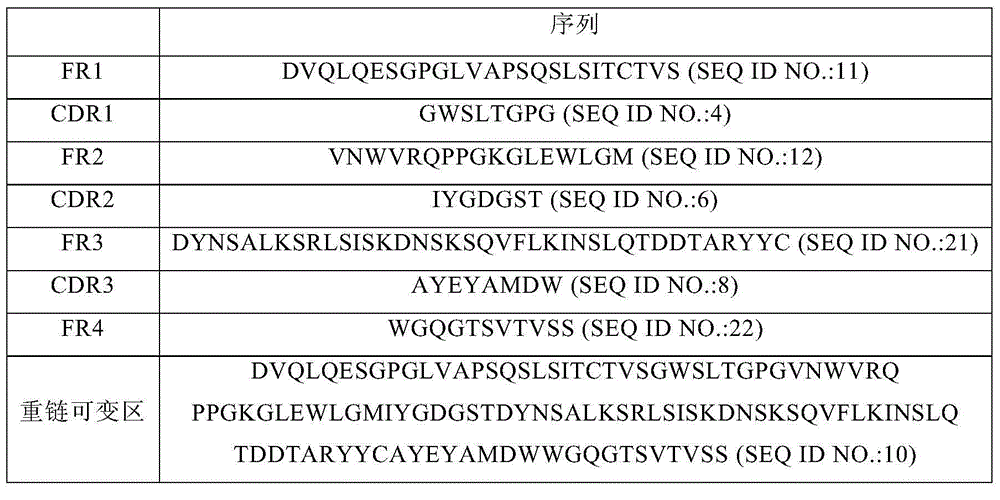
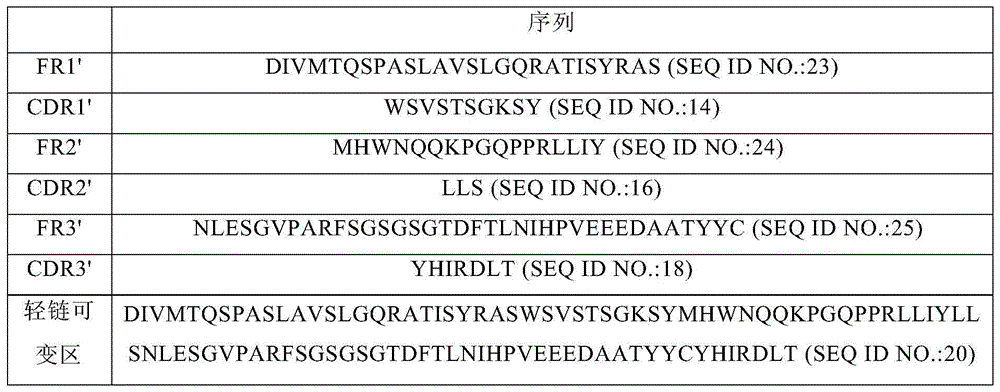
PD-1 monoclonal antibody and application thereof
An antibody and carrier technology, applied in the field of biomedicine, can solve problems such as unsatisfactory antibody performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] Preparation of monoclonal antibodies
[0142] Antibodies of the present invention can be prepared by various techniques known to those skilled in the art. For example, an antigen of the invention may be administered to an animal to induce the production of monoclonal antibodies. Monoclonal antibodies can be prepared using hybridoma technology (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J. Immunol. 6:511, 1976; Kohler et al., Eur.J.Immunol. 6:292,1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981) or can be prepared by recombinant DNA methods (US Patent No. 4,816,567).
[0143] Representative myeloma cells are those that fuse efficiently, support stable high-level production of antibody by selected antibody-producing cells, and are sensitive to culture medium (HAT medium matrix), including myeloma cell lines, such as murine Myeloma cell lines, including those derived from MOPC-21 and MPC-11 mouse tumors (avai...
Embodiment 1
[0172] The preparation of embodiment 1 monoclonal antibody
[0173] 1.1 Animal immunity
[0174] 6-8 weeks old female BALB / C mice were taken, and the immunogen was recombinantly expressed PD-1 protein 293T cells, the amino acid sequence was as follows:
[0175] DSPDRPWNPPTFSPALLVVTEGDNATFTCSFSNTSESFVLNWYRMSPSNQTDKLAAFPEDRSQPGQDCRFRVTQLPNGRDFHMSVVRARRNDSGTYLCGAISLAPKAQIKESLRAELRVTERRAEVPTAHPSPSPRPAGQFQTLVVGVVGGLLGSLVLLVWVLAVICSRAARGTIGARRTGQPLKEDPSAVPVFSVDYGELDFQWREKTPEPPVPCVPEQTEYATIVFPSGMGTSSPARRGSADGPRSAQPLRPEDGHCSWPL(SEQIDNO.:1),免疫程序见表2。 Before each immunization, blood was collected from the tail of the mice, and 293T cells expressing PD-1 protein by gene recombination were used as the detection antigen coating indirect ELISA method to detect the titer of mouse serum. Splenocytes were taken for fusion when the serum titer titer of the immunized mice reached the maximum and no longer increased.
[0176] Table 2 Mouse immunization program
[0177] immune time
...
Embodiment 2
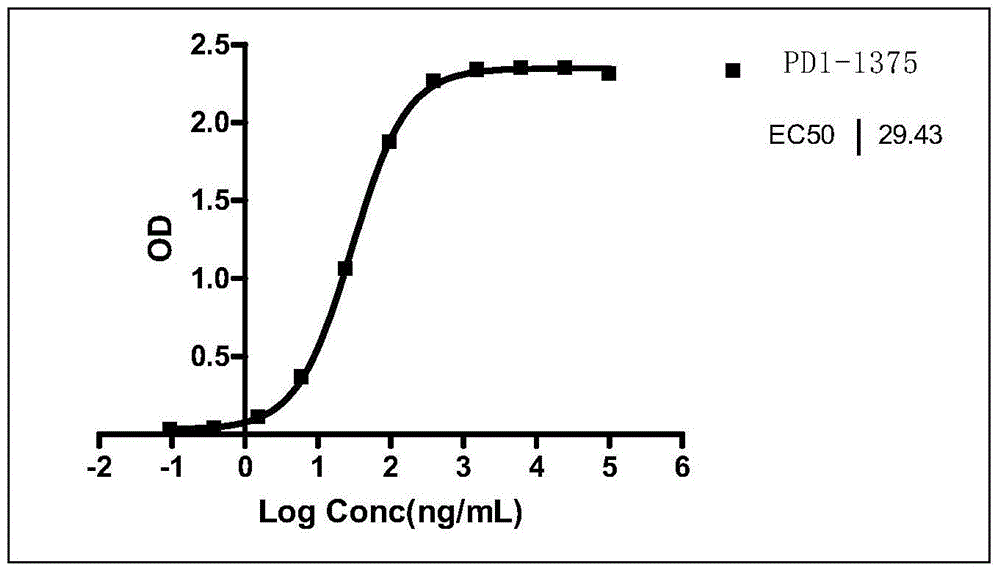
[0188] Example 2 Affinity analysis of PD1-1375 to soluble human PD-1
[0189] The soluble antigen protein PD1-Fc was dissolved in PBS at a concentration of 5 μg / ml for antigen coating;
[0190] Antibody incubation with PD1-1375 in different concentration gradients;
[0191] Dissolve goat anti-mouse IgG-HRP (ProSci) in diluent (1%BSA+0.05%PBST20) at 1:5000, incubate with secondary antibody, and develop color; the experimental results are as follows figure 1 As shown, the EC50 of the PD1-1375 antibody was obtained = 29.4 ng / mL.
[0192] PD1-Fc is composed of the extracellular domain of human PD1 molecule plus the Fc fragment of human IgG1, and its amino acid sequence is as follows:
[0193] DSPDRPWNPPTFSPALLVVTEGDNATFTCSFSNTSESFVLNWYRMSPSNQTDKLAAFPEDRSQPGQDCRFRVTQLPNGRDFHMSVVRARRNDSGTYLCGAISLAPKAQIKESLRAELRVTERRAEVPTAHPSPSPRPAGQFQTLVVGVVGGLLGSLVLLVWVLAVICSRAARGTIGARRTGQPLKEDPSAVPVFSVDYGELDFQWREKTPEPPVPCVPEQTEYATIVFPSGMGTSSPARRGSADGPRSAQPLRPEDGHCSWPL P KSCDKTHTCPPCPAPELLGGPSVF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com