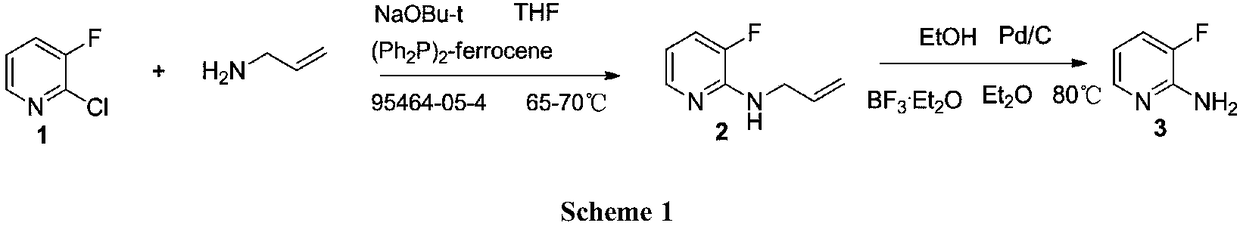
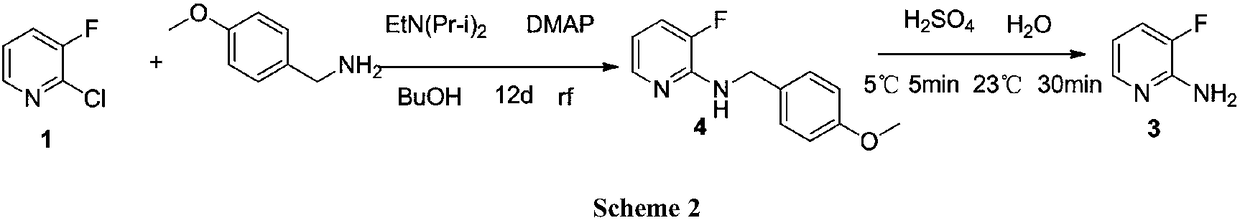
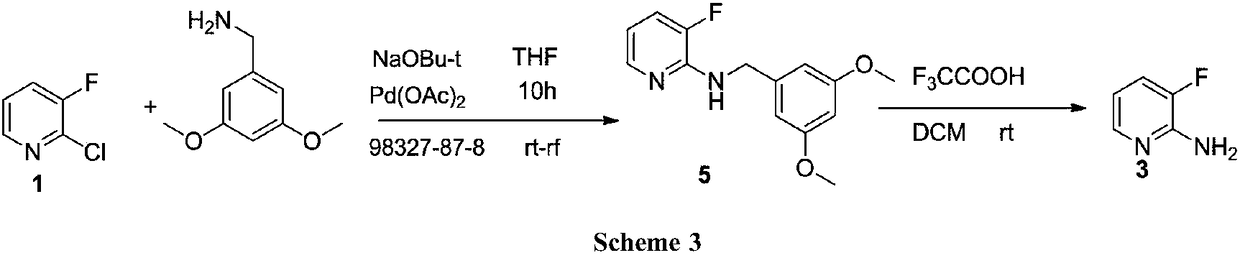
A kind of preparation technology of 2-amino-3-fluoropyridine
A technology of fluoropyridine and amino, which is applied in the field of preparation technology of 2-amino-3-fluoropyridine, can solve the problems of poor environmental friendliness, cumbersome post-processing, high safety risk, etc., and achieve high product yield and suppress by-products Formation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A. 2-Amino-3-fluoro-5-chloropyridine
[0027] 2,3-Difluoro-5-chloropyridine (100 g, 0.669 mol, 1 eq) and ammonia (1.125 L, 8.025 mol, 12 eq) were added to the autoclave. After sealing, the reaction was carried out at 120 °C for 20 h. After the reaction was completed, a pale yellow solid was precipitated after cooling. The filter cake was washed with water by suction filtration, and the filtrate was extracted with ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate. A small amount of petroleum ether was slurried, filtered with suction, and the obtained filter cake and the separated filter cake were collected simultaneously to obtain 83.61 g of compound 2-amino-3-fluoro-5-chloropyridine with a yield of 85.32%.
[0028] 1 H-NMR (DMSO): 6.442 (s, 2H,); 7.577-7.609 (d, 1H); 7.778-7.785 (d, 1H).
[0029] M / S:147.1[M+H] + .
[0030] B. Preparation of 2-amino-3-fluoropyridine (3)
[0031] In a 1000ml single-neck flask, 2-amino-3-fluoro-5...
Embodiment 2
[0037] A. 2-Amino-3-fluoro-5-chloropyridine
[0038] 2,3-Difluoro-5-chloropyridine (100 g, 0.669 mol) and ammonia (1.5 L, 10.7 mol) were added to the autoclave. After sealing, the reaction was carried out at 140 °C for 20 h. After the reaction was completed, a pale yellow solid was precipitated after cooling. The filter cake was washed with water, and the filtrate was extracted with ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate. The organic phase was evaporated under reduced pressure and used A small amount of petroleum ether was slurried, filtered with suction, and the obtained filter cake and the separated filter cake were collected simultaneously to obtain 83.55 g of compound 2-amino-3-fluoro-5-chloropyridine with a yield of 85.26%.
[0039] 1 H-NMR (DMSO): 6.442 (s, 2H,); 7.577-7.609 (d, 1H); 7.778-7.785 (d, 1H).
[0040] M / S:147.1[M+H] + .
[0041] B. Preparation of 2-amino-3-fluoropyridine (3)
[0042] In a 1000ml single-nec...
Embodiment 3
[0048] A. 2-Amino-3-fluoro-5-chloropyridine
[0049] 2,3-Difluoro-5-chloropyridine (100 g, 0.669 mol) and ammonia (1.5 L, 10.7 mol) were added to the autoclave. After sealing, react at 120°C for 24 hours. After the reaction is completed, a light yellow solid precipitates out after cooling. Filter with suction, wash the filter cake with water, extract the filtrate with ethyl acetate, combine the organic layers and dry them with anhydrous sodium sulfate. Evaporate the organic phase under reduced pressure and use Slurry with a small amount of petroleum ether, filter with suction, and collect the obtained filter cake and the precipitated filter cake simultaneously to obtain 83.82 g of the compound 2-amino-3-fluoro-5-chloropyridine, with a yield of 85.53%.
[0050] 1 H-NMR (DMSO): 6.442 (s, 2H,); 7.577-7.609 (d, 1H); 7.778-7.785 (d, 1H).
[0051] M / S:147.1[M+H] + .
[0052] B. Preparation of 2-amino-3-fluoropyridine (3)
[0053] Dissolve 2-amino-3-fluoro-5-chloropyridine (50g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com