Compositions and methods for modulating farnesoid x receptors
A compound, technology of alkyl, applied in the field of composition for regulating farnesoid X receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
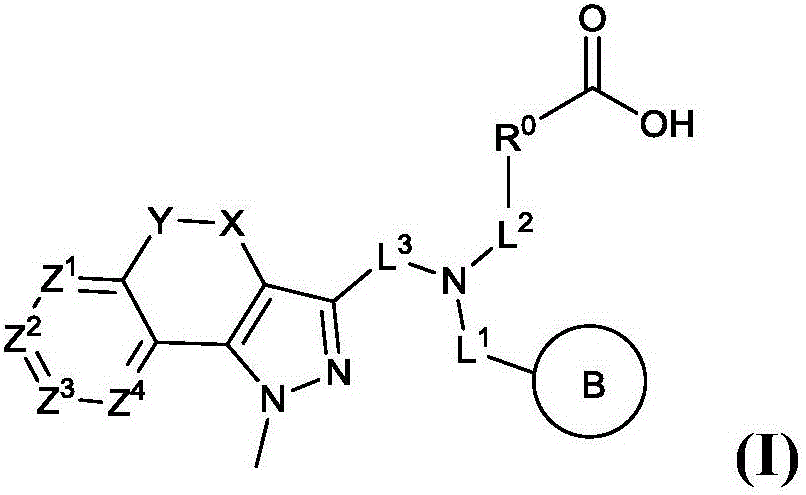
[0246] Embodiment 1. A compound of formula (I) or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof,
[0247]
[0248] in:
[0249] R 0 for Ring A or C 1-6 Alkyl; where
[0250] Ring A is an aryl group; a 5-10 membered heteroaryl group containing 1-3 N, O or S heteroatoms; a 4-6 membered heterocycle containing 1-2 N, O or S heteroatoms; or C 3-7 Cycloalkyl; each of them is unsubstituted or replaced by 1-2 each represented by R 2 Substituents independently represented by substituents; where L 3 and R 0 Can be attached to the same and different ring atoms of ring A; C 1-6 Alkyl is optionally replaced by 1-2 C 1-6 Alkyl substitution;
[0251] Ring B is aryl; 5-10 membered heteroaryl containing 1-3 N, O or S heteroatoms; 4-6 membered heterocyclic ring containing 1-2 N, O or S heteroatoms; or C 3-7 Cycloalkyl; each of them is unsubstituted or replaced by 1-2 each represented by R 2 substituent substitution by independent representatives;
[0252] ...
Embodiment approach 2
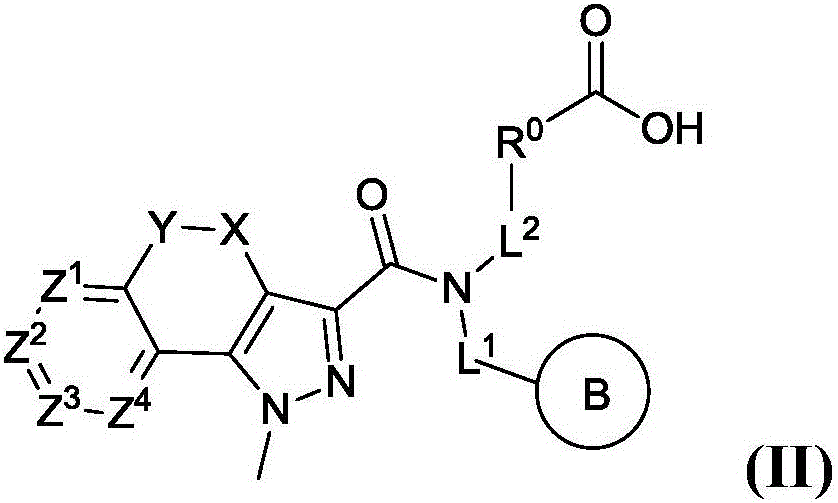
[0261] Embodiment 2. The compound of Embodiment 1, or a salt, tautomer or stereoisomer thereof, wherein said compound is represented by formula (II),
[0262]
[0263] in:
[0264] R 0 for Ring A or C 1-6 Alkyl; where:
[0265] Ring A is selected from phenyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl and C 3-7 Cycloalkyl, each of which is unsubstituted or replaced by 1 to 3 each represented by R 2 Substituents independently represented by substituents; where L 3 and R 0 Can be attached to the same and different ring atoms of ring A; C 1-6 Alkyl is optionally replaced by 1-2 C 1-6 Alkyl substitution;
[0266] Ring B is selected from phenyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, 1H-indolyl and C 3-7 Cycloalkyl, each of which is unsubstituted or replaced by 1-2 each represented by R 2 substituent substitution by independent representatives;
[0267] X is (CR 4 R 5 );
[0268] Y is O, (CR 4 R 5 ) or *O(CR 4 R 5 ), where "*" indicates the point of at...
Embodiment approach 3
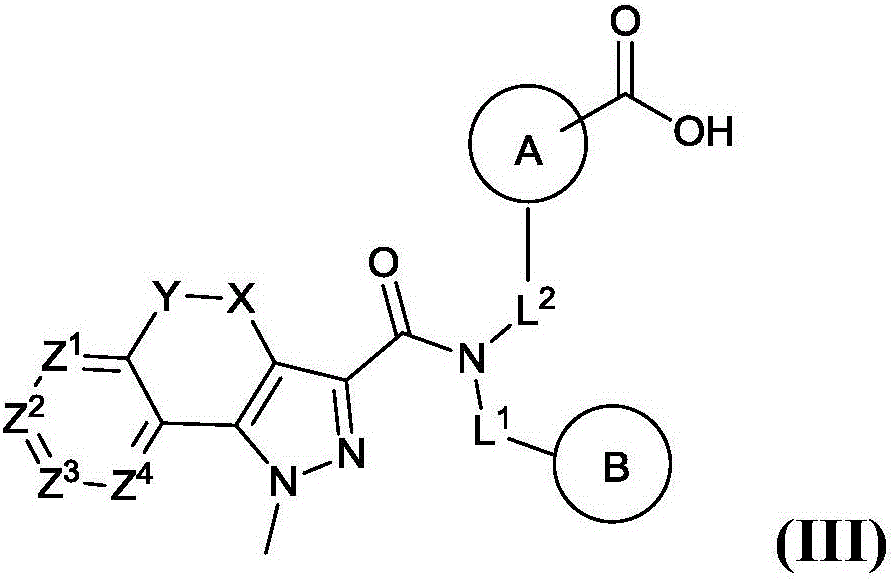
[0275] Embodiment 3. The compound of Embodiment 1 or 2, or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof, wherein R 0 selected from* 3 -CH 2 C(CH 3 ) 2 -,* 3 -CH 2 CH(CH 3 )-and* 3 -cyclopropane-1,1-diyl-, where "* 3 " means R 0 with L 2 connection point.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com