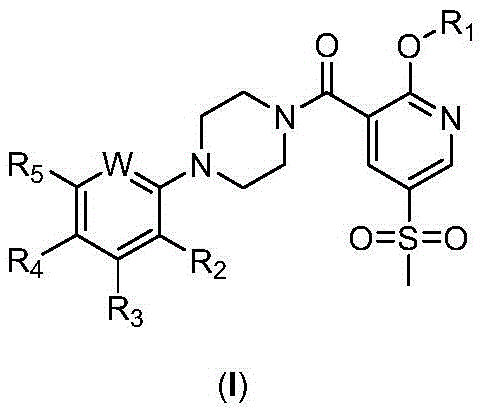
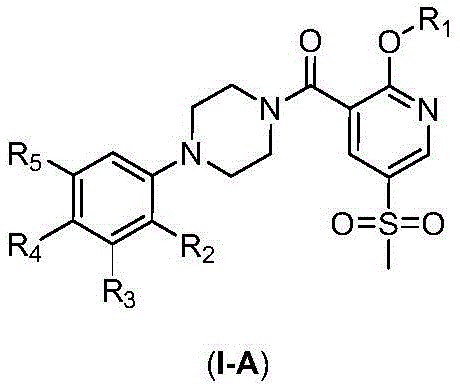
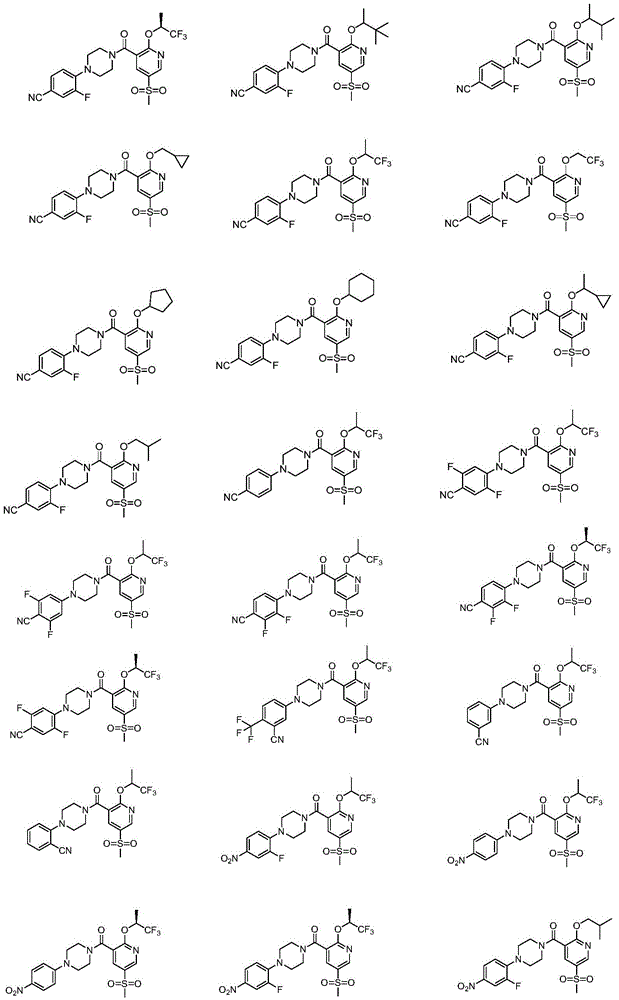
2-Substituted-oxy-5-methylsulfonyl pyridine piperazine acidamide analogue and preparation method and application thereof
A technology of thiamphenicol pyridine piperazine amide and analogues, applied in the field of 2-substituted oxy-5-thiamphenicol pyridine piperazinamide analogues and their preparation, capable of solving negative symptoms and no improvement in cognitive symptoms And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] 1. Preparation of important intermediates
[0094] 1. Preparation of intermediate 1: 1-(3-fluoro-5-(trifluoromethyl)pyridin-2-yl)piperazine
[0095]
[0096] 2,3-5-Trifluoromethylpyridine (15.0g, 0.08mmoL) and potassium carbonate (33.9g, 0.24g) were dissolved in tetrahydrofuran, piperazine (35.2g, 0.4mmoL) was added in batches under an ice-water bath, and then Stir at room temperature for 1 hour, then extract with 40 mL of toluene, wash three times with water, dry the organic phase over sodium sulfate, filter and evaporate the solvent to obtain 1-(3-fluoro-5-(trifluoromethyl)pyridin-2-yl ) piperazine (10 g).
[0097] 2. Preparation of intermediate 2: 1-(2-fluoro-4-(trifluoromethyl)phenyl)piperazine
[0098]
[0099] Weigh 2-fluoro-4-trifluoromethylbromobenzene (243mg, 1.0mmol), piperazine (95mg, 1.1mmol), bis(tri-tert-butylphosphine)palladium (51mg, 0.10mmol) and cesium carbonate (489mg , 1.5mmol), added to 5mL of toluene, pumped and ventilated, nitrogen protec...
Embodiment 1
[0293] (4-(3-fluoro-5-(trifluoromethyl)pyridin-2-yl)piperazin-1-yl)(5-(methylsulfonyl)-2-((1,1,1-trifluoro Propan-2-yl)oxo)pyridin-3-yl)methanone
[0294]
[0295] (5-bromo-2-((1,1,1-trifluoropropan-2-yl)oxo)pyridin-3-yl)(4-(3-fluoro-5-(trifluoromethyl)pyridine- 2-yl)piperazin-1-yl)methanone (150mg, 0.28mmol), sodium methanesulfinate (198mg, 1.65mmol), cuprous iodide (26mg, 0.14mmol) and sodium proline (38mg , 0.28mmol), dissolved in 3mL dimethyl sulfoxide, pumped, nitrogen protection. Heat at 120°C to react overnight, cool to room temperature after the reaction is complete, and add water to quench. The reaction mixture was separated by reverse-phase column chromatography to obtain a white solid (4-(3-fluoro-5-(trifluoromethyl)pyridin-2-yl)piperazin-1-yl)(5-(methylsulfonyl) -2-((1,1,1-trifluoropropan-2-yl)oxo)pyridin-3-yl)methanone (60 mg, 40%).
[0296] LC-MS:t R =4.39min, [M+H] + =545.0;
[0297] 1 HNMR (400MHz, CDCl 3 )δ8.69(d, J=2.4Hz, 1H), 8.19(d, J=0.9Hz, 1H)...
Embodiment 2
[0300] (S)-(4-(3-fluoro-5-(trifluoromethyl)pyridin-2-yl)piperazin-1-yl)(5-(methylsulfonyl)-2-((1,1, 1-trifluoropropan-2-yl)oxo)pyridin-3-yl)methanone
[0301]
[0302] The preparation method refers to Example 1.
[0303] LC-MS:t R =3.03min, [M+H] + =545.0;
[0304] 1 HNMR (400MHz, CDCl 3 )δ8.69(d,J=2.4Hz,1H),8.18(s,1H),8.13(s,1H),7.37(dd,J=13.0,1.7Hz,1H),5.93(brs,1H), 3.99–3.43(m,6H),3.29(brs,2H),3.06(s,3H),1.47(brs,3H);
[0305] 19 FNMR (376MHz, CDCl 3 ) δ -61.23(s), -78.01(s), -127.72(d, J=11.7Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com