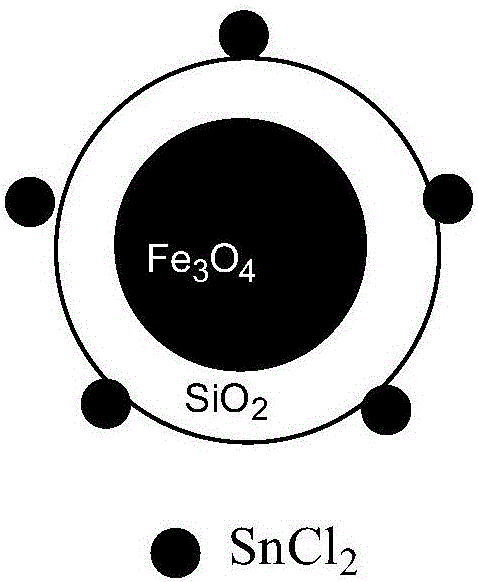
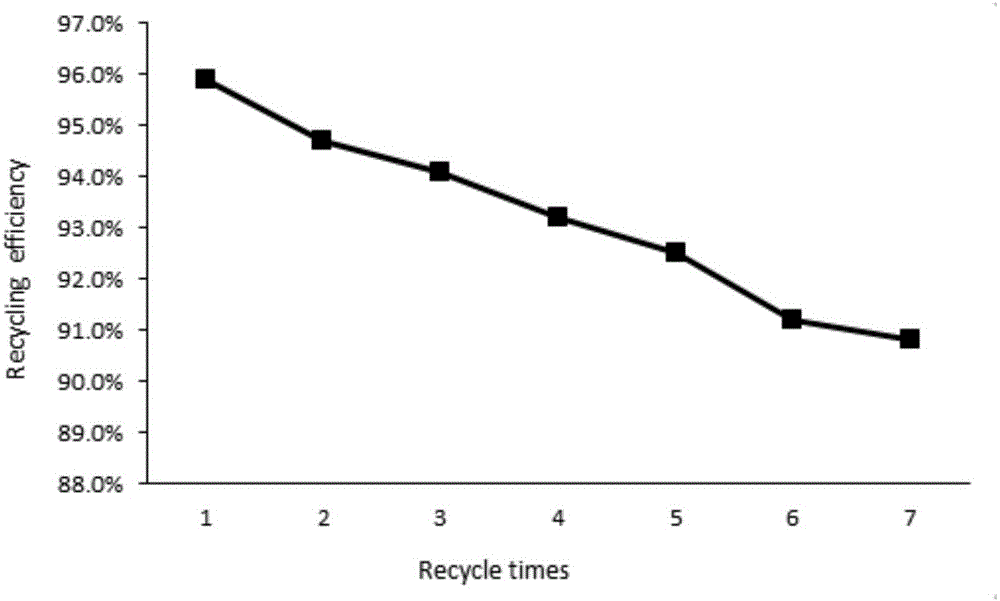
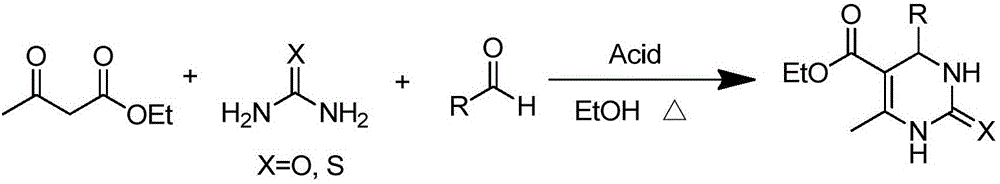
Method for synthesizing 3,4-dihydropyrimidine-2-one derivative
A technology for dihydropyrimidine and derivatives, applied in the field of synthesizing 3,4-dihydropyrimidin-2-one derivatives, which can solve the problems of difficult recovery and high recovery cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 50 mL round bottom flask, add benzaldehyde (3.0 mmol), ethyl acetoacetate (3.0 mmol), urea (3.6 mmol), 4 mL ethanol, magnetic nanoparticles supported SnCl 2 Catalyst 0.3g, reacted at a reaction temperature of 75°C for 5h. After judging by thin-layer chromatography that the reaction is complete, the catalyst is held by a magnet, and the reaction solution is poured out while it is hot. After cooling to room temperature, 10 mL of cold water was added to precipitate a solid. Filter, wash the solid with 3 mL of cold ethanol, and recrystallize the solid with ethanol. 0.751 g of 4-phenyl-6-methyl-5-(ethoxycarbonyl)-3,4-dihydropyrimidin-2-one was obtained, with a yield of 96.2% and a content of 95.6%. 1 HNMR(DMSO-d6)δ: 9.17(s,1H),7.72(s,1H),7.32(t,2H,J=7.2Hz),7.23-7.25(m,3H),5.14(d,1H,J =3.2Hz), 3.96-4.01(m, 2H), 2.25(s, 3H), 1.09(t, 3H, J=7.2Hz); 13 CNMR(DMSO-d6)δ: 165.81, 152.58, 145.34, 128.84, 127.71, 126.71, 99.77, 59.63, 54.44, 18.23, 14.54.
Embodiment 2
[0029] In a 50 mL round bottom flask, add 3-nitrobenzaldehyde (3.0 mmol), ethyl acetoacetate (3.0 mmol), urea (3.6 mmol), 4 mL ethanol, magnetic nanoparticles supported SnCl 2 Catalyst 0.3g was reacted at a reaction temperature of 75°C for 7h. Use thin-layer chromatography to judge that the reaction is complete, use a magnet to hold the catalyst, and pour out the reaction solution while it is hot. After cooling to room temperature, 10 mL of cold water was added to precipitate a solid. Filter, wash the solid with 3 mL of cold ethanol, and recrystallize the solid with ethanol. 0.806 g of 4-(3-nitrophenyl)-6-methyl-5-(ethoxycarbonyl)-3,4-dihydropyrimidin-2-one was obtained, with a yield of 88.0% and a content of 97.0%. 1 HNMR(DMSO-d6)δ: 9.37(s,1H), 8.10-8.15(m,2H), 7.91(s,1H), 7.64-7.72(m,2H), 5.32(d,1H, J=3.2Hz ),4.00-4.02(m,2H),2.29(s,3H),1.11(s,3H); 13 CNMR(DMSO-d6)δ: 165.53, 152.25, 149.87, 148.24, 147.47, 133.45, 130.67, 122.79, 121.48, 98.84, 59.85, 54.04, 18.31, 14.46....
Embodiment 3
[0031] In a 50 mL round bottom flask, add 2-chlorobenzaldehyde (3.0 mmol), ethyl acetoacetate (3.0 mmol), urea (3.6 mmol), 4 mL ethanol, magnetic nanoparticles supported SnCl 2 Catalyst 0.3g, reacted at a reaction temperature of 75°C for 6h. Use thin-layer chromatography to judge that the reaction is complete, use a magnet to hold the catalyst, and pour out the reaction solution while it is hot. After cooling to room temperature, 10 mL of cold water was added to precipitate a solid. Filter, wash the solid with 3 mL of cold ethanol, and recrystallize the solid with ethanol. 0.778 g of 4-(2-chlorophenyl)-6-methyl-5-(ethoxycarbonyl)-3,4-dihydropyrimidin-2-one was obtained, with a yield of 88.0% and a content of 96.7%. 1 HNMR (DMSO-d6) δ: 9.25(s, 1H), 7.68(s, 1H), 7.39(d, 1H, J=7.6Hz), 7.31(t, 2H, J=3.6Hz), 7.26(d, 1H, J=7.6Hz), 5.63(d, 1H, J=3.2Hz), 3.87-3.92(m, 2H), 2.30(s, 3H), 0.99(t, 3H, J=7.2Hz); 13CNMR(DMSO-d6)δ: 165.43, 151.77, 149.73, 142.19, 132.17, 129.82, 129.52, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com