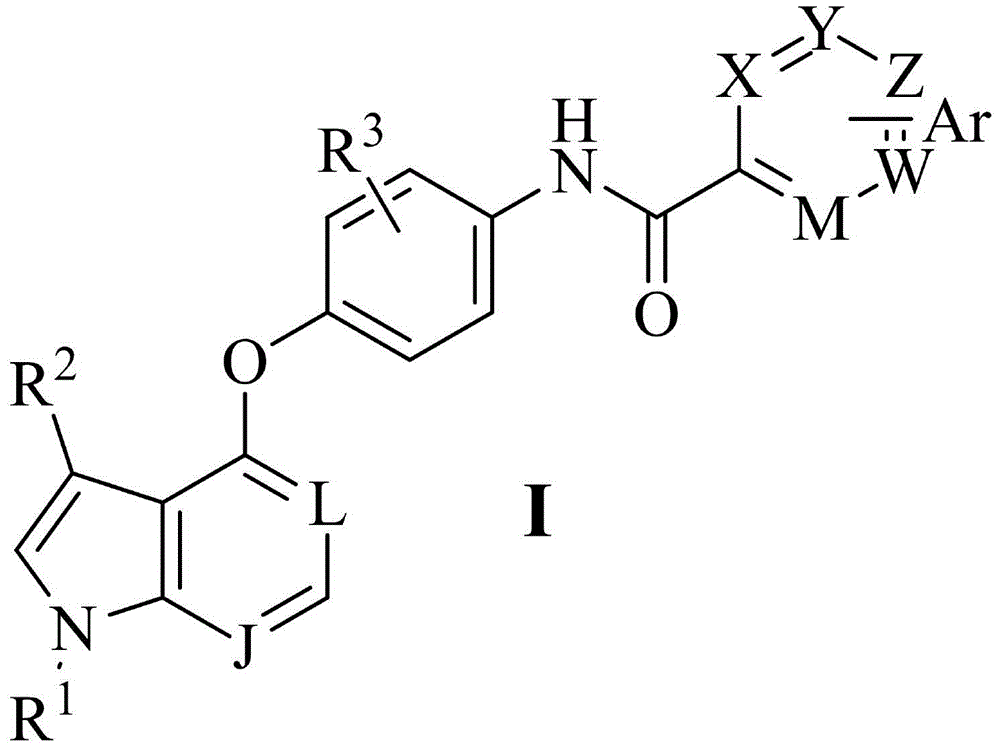
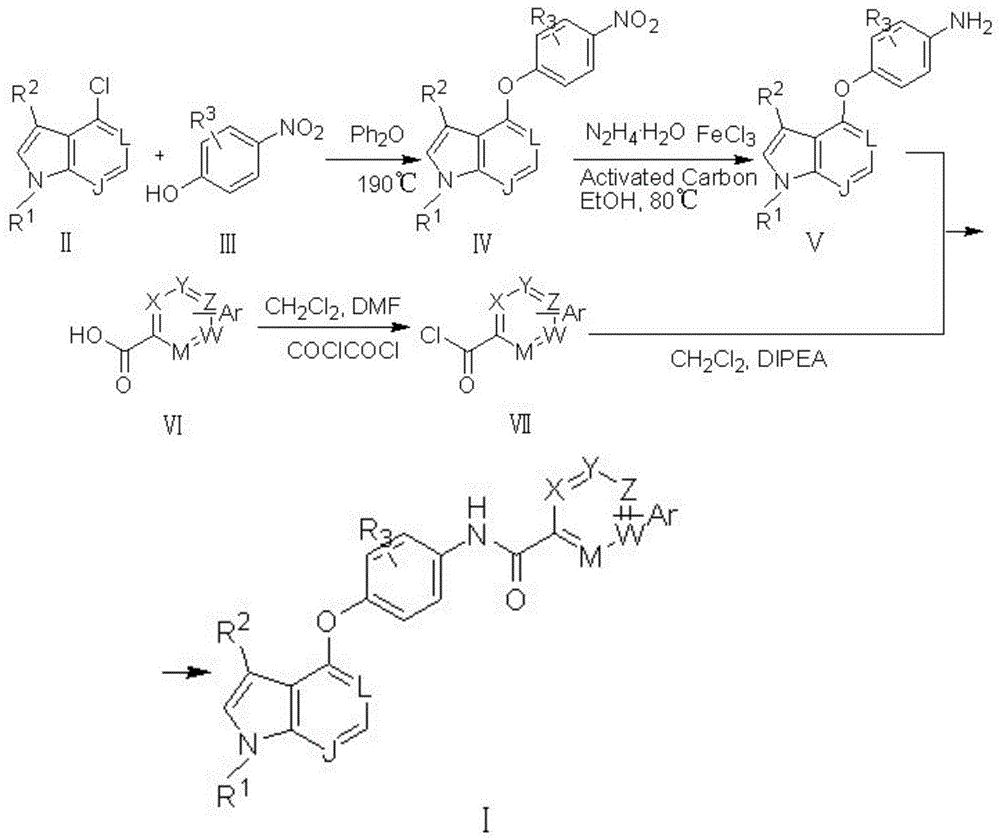
Pyrrolopyridine compounds containing biaryl amide structure, preparation method and applications thereof
A technology of pyrrolopyridine and arylamide, which is applied in the field of pyrrolopyridine compounds containing biarylamide structure and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
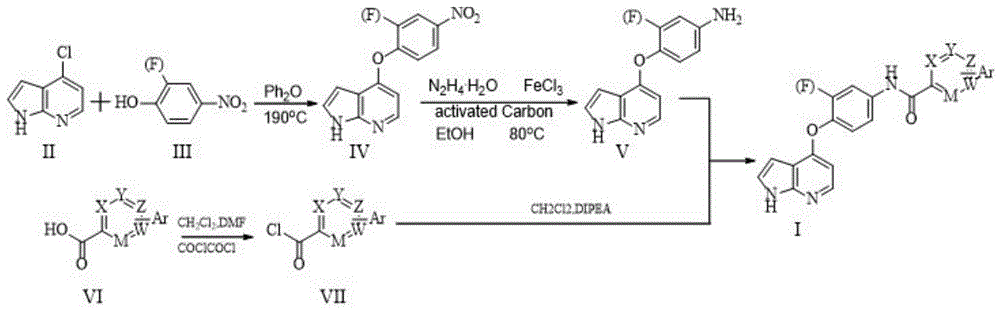
[0149] N-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluorophenyl)-4-phenylpyridineamide
[0150] Step A4-Synthesis of (2-fluoro-4-nitrophenoxy)-1H-pyrrolo[2,3-b]pyridine (IV)
[0151] In a 250mL three-necked flask, preheat diphenyl ether (51.843g) until completely dissolved, then add 4-chloro-7-azaindole (9.996g) and 2-fluoro-4-nitrophenol (16.862 g), heat up to 190°C and react for about 1 hour. When the temperature rises to about 130°C, all solids dissolve and the solution appears light yellow. As the temperature rises, the color of the solution deepens and the appearance is dark brown. After the reaction was completed, the reaction liquid was cooled, slowly added dropwise into 400 mL of ethyl acetate and stirred for 2 h, the precipitated solid was suction filtered, and the filter cake was dried to obtain 8.285 g of khaki powder with a yield of 46.3%.
[0152] Step B Synthesis of 4-((1H-pyrrolo[2,3-b]pyridin-4-yl)oxy)-3-fluoroaniline (V)
[0153] Add 100mL of ethanol to a 250m...
Embodiment 2
[0162] N-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluorophenyl)-4-m-tolylpyridinamide
[0163] ESI-MS[M+H](m / z):439.5
[0164] 1 HNMR(400MHz,DMSO)δ11.85(s,1H),11.13(s,1H),8.90(d,J=4.5Hz,1H),8.51(s,1H),8.23(t,J=11.2Hz, 1H), 8.18(d, J=5.3Hz, 1H), 8.11(s, 1H), 7.99(d, J=8.2Hz, 1H), 7.82(s, 1H), 7.78(d, J=7.6Hz, 1H), 7.57(d, J=7.3Hz, 1H), 7.54–7.50(m, 1H), 7.49–7.42(m, 2H), 6.50(d, J=5.1Hz, 1H), 6.37(s, 1H) ),2.53(s,3H).
Embodiment 3
[0166] N-(4-(1H-pyrrolo[2,3-b]pyridin-4-yloxy)-3-fluorophenyl)-4-p-tolylpyridinamide
[0167] ESI-MS[M+H](m / z):439.5
[0168] 1 ( d,J=13.1Hz,1H),8.18(d,J=5.2Hz,1H),8.10(d,J=3.2Hz,1H),7.99(d,J=8.7Hz,1H),7.91(d, J=7.6Hz, 1H), 7.52(d, J=6.8Hz, 1H), 7.48(d, J=7.2Hz, 3H), 6.49(d, J=5.1Hz, 1H), 6.36(s, 1H) ,6.36(s,1H),2.49(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com