Crystal form of AHU377 as well as preparation method and application thereof
A crystal form and crystallization technology, which is applied in the field of AHU377 crystal form and its preparation, can solve problems such as cumbersome operation and unfavorable process quality control, and achieve the effects of simplifying the process flow, avoiding the introduction of impurity ions, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Preparation method of AHU377 crystal form I:
[0075] Dissolve 801.8mg of AHU377 in 5mL of toluene solution, add 5mL of n-heptane, and then place it at 4℃ and stir overnight to get it.
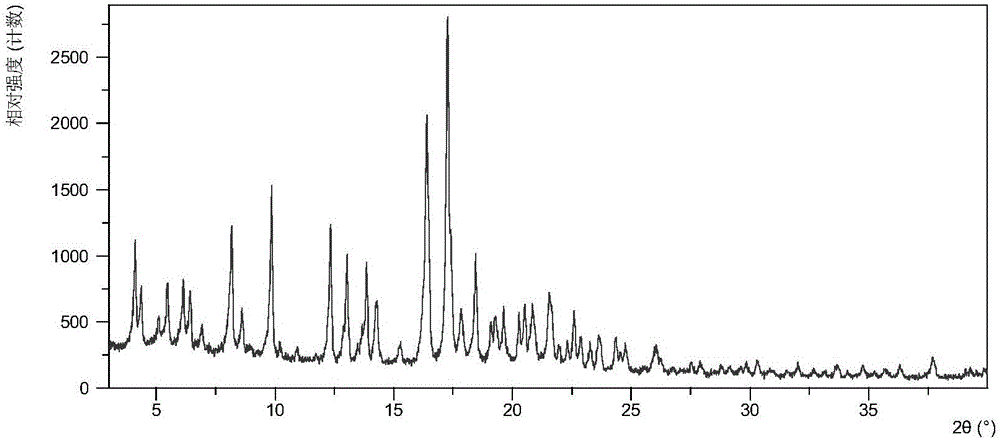
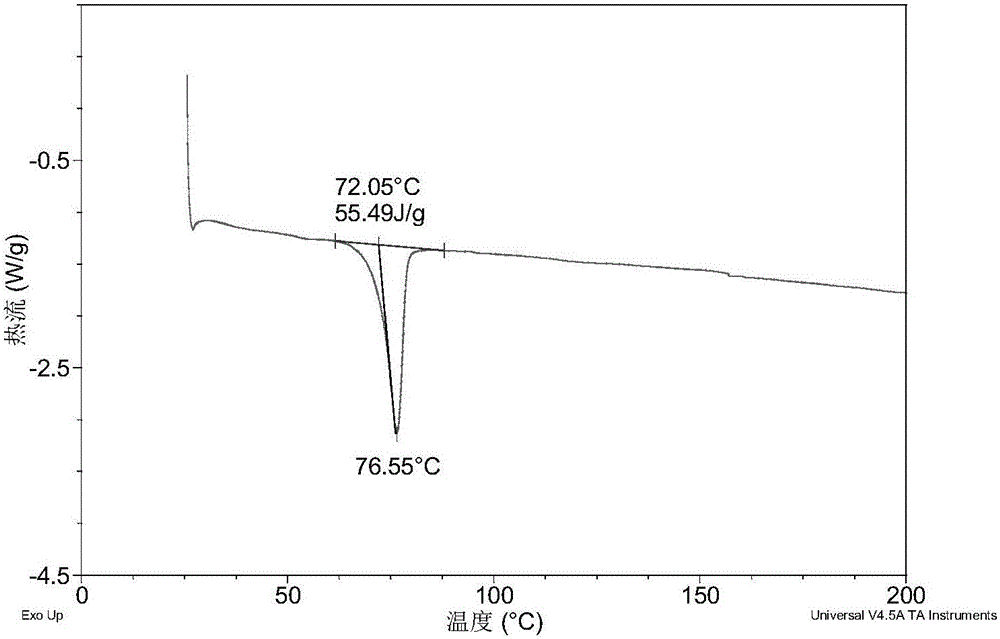
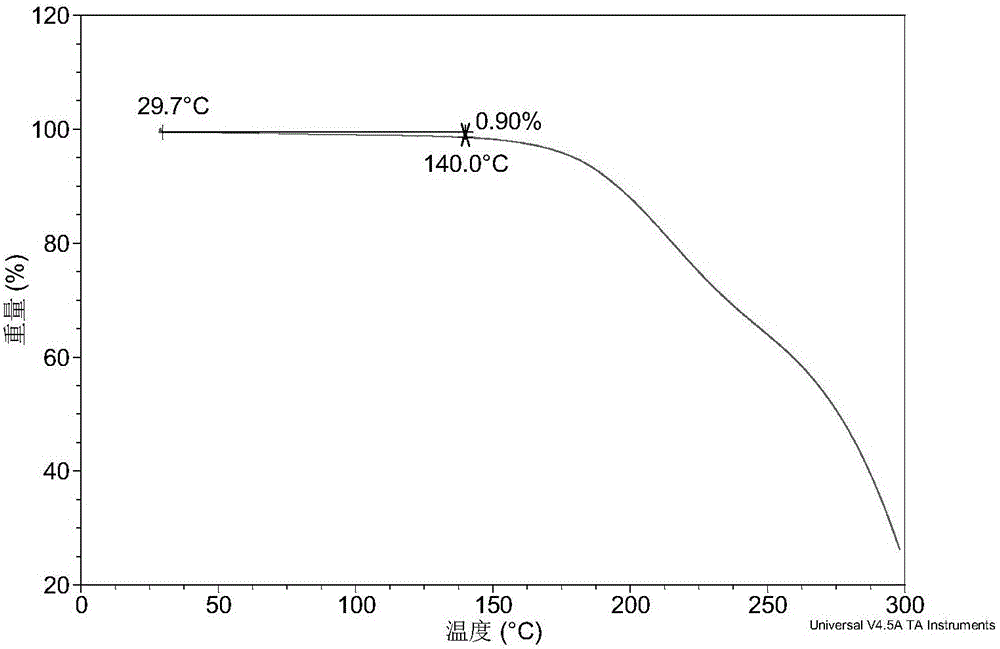
[0076] Table 1 shows the X-ray powder diffraction data of the crystal form I obtained in this example. Its XRPD diagram is like figure 1 , Its DSC graph is like figure 2 , The TGA diagram is as image 3 .
[0077] Table 1
[0078]
[0079]
[0080]
Embodiment 2
[0082] Preparation method of AHU377 crystal form I:
[0083] Dissolve 510 mg of AHU377 (with an initial purity of 98.74%) in 3 mL of toluene solution, gradually add 3 mL of n-heptane after dissolving, add AHU377 crystal form I as a seed crystal during the process, and place it at room temperature (25°C) and stir to obtain . The final crystal form AHU377 has a purity of 99.64%. The preparation of AHU377 crystal form I can play a role in the purification of raw materials. Through the HPLC purity test, it can be found that the purity is increased from 98.74% to 99.64%, and the purification effect is significant.
[0084] The X-ray powder diffraction data of the crystal form obtained in this example is shown in Table 2.
[0085] Table 2
[0086]
[0087]
Embodiment 3
[0089] Preparation method of AHU377 crystal form I:
[0090] Dissolve 2.02g of AHU377 (with an initial purity of 98.74%) in 20mL of toluene solution, and gradually add 10mL of n-heptane after dissolving. During the process, add AHU377 crystal form I as a seed crystal, and place it at room temperature (25°C) and stir. available. The final crystal form AHU377 has a purity of 99.69%.
[0091] Table 3 shows the X-ray powder diffraction data of the crystal form obtained in this example.
[0092] table 3
[0093]
[0094]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com