Co-crystals of CDK inhibitor and MEK inhibitor and preparation method of co-crystals
An inhibitor, MEK162 technology, applied in the field of chemical medicine, can solve the problems such as the therapeutic effect of co-crystal made of two active ingredients of medicine has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0108] In the preparation method of the crystal form of the present invention:
[0109] The "room temperature" refers to 15-25°C.
[0110] The "stirring" is accomplished by conventional methods in the art, such as magnetic stirring or mechanical stirring, and the stirring speed is 50-1800 rpm, preferably 300-900 rpm.
[0111] The "separation" is accomplished by conventional methods in the art, such as centrifugation or filtration. The operation of "centrifugation" is: put the sample to be separated in a centrifuge tube, and centrifuge at a speed of 10,000 rpm until all the solids sink to the bottom of the centrifuge tube.
[0112] Unless otherwise specified, the "drying" can be carried out at room temperature or higher. The drying temperature is from room temperature to about 60°C, or to 40°C, or to 50°C. The drying time can be 2-48 hours, or overnight. Drying is carried out in a fume hood, forced air oven or vacuum oven.
[0113] In the present invention, "crystal" or "c...
Embodiment 1
[0154] The preparation method of crystal form I:
[0155] Put 26.6 mg of MEK162 and 4 mL of acetonitrile / water (v:v=19:1) at 50°C and stir for 30 minutes, add 16.0 mg of LEE011, continue stirring overnight and slowly cool down to 20°C, and collect the solid to obtain it.
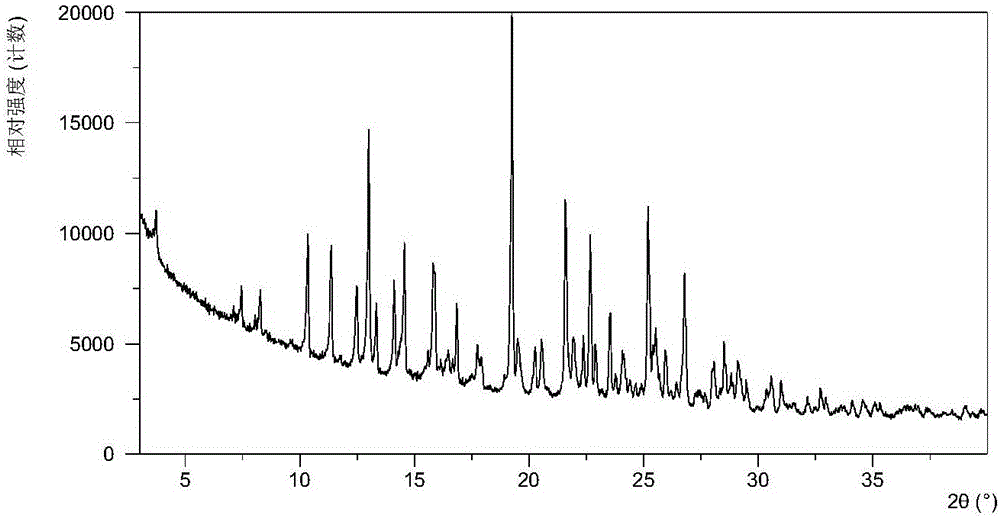
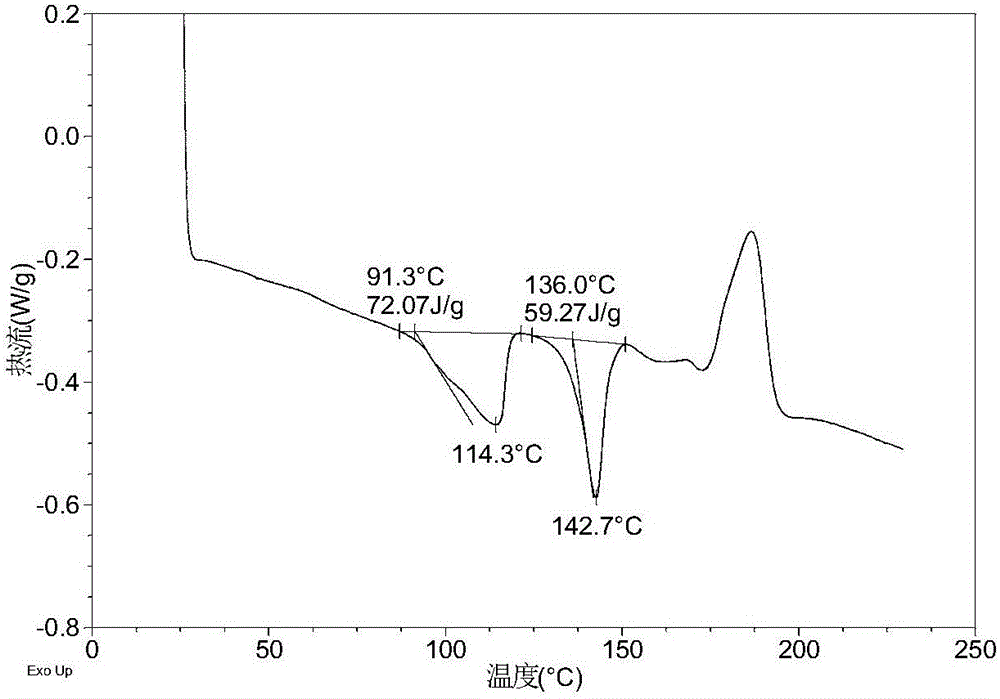
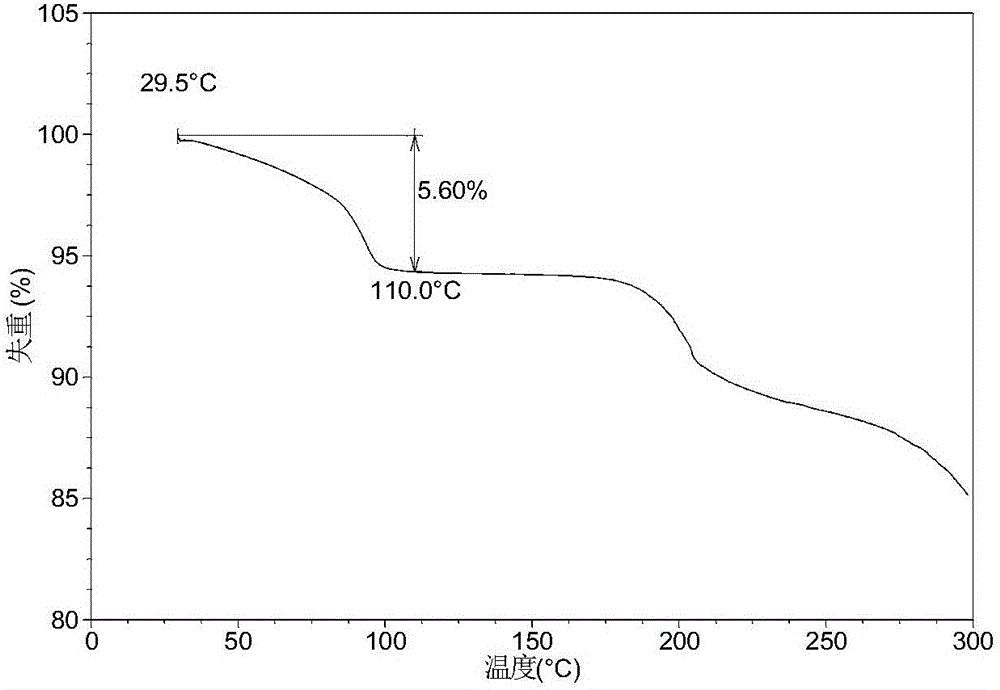
[0156] Table 1 shows the X-ray powder diffraction data of the crystal forms obtained in this example. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 . From figure 2 It can be seen that the crystal form I of this example begins to appear the first endothermic peak when heated to around 86-95°C, and the second endothermic peak begins to appear around 130-142°C. From image 3 It can be seen that the crystal form I of this example has a weight loss gradient of about 5-6% when heated to around 110°C.
[0157] Table 1
[0158]
[0159]
Embodiment 2
[0161] The preparation method of crystal form I:
[0162] Mix 9.7mg of MEK162 and 1mL of acetonitrile / water (v:v=1:1) at room temperature (25±3°C) and stir for 30 minutes, add 10.0mg of LEE011, and continue stirring overnight to obtain.
[0163] The X-ray powder diffraction data of the crystal forms obtained in this example are shown in Table 2.
[0164] Table 2
[0165]
[0166]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com