Solid compositions of triglycerides and uses thereof
一种固体组合物、三酸甘油酯的技术,应用在药物组合、酐/酸/卤化物有效成分、含有效成分的医用配制品等方向,能够解决影响、1型葡萄糖转运体缺乏疗效等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Table 5 demonstrates the high purity of exemplary triheptanoin oil samples and solid samples comprising triheptanoin oil according to the present invention.
[0155] Additionally, the stability of an exemplary solid composition comprising triheptanoin oil (solid sample 2 in Table 5) was tested. Measurements were taken after storage in double LDPE (low density polyethylene) bags in 60 cc induction sealed HDPE (high density polyethylene) bottles at 25°C / 60% RH (relative humidity) and the results are shown in Table 6. Measurements were also carried out at 40°C / 75%RH in 60cc induction sealed HDPE bottles after storage in double LDPE bags and the results are shown in Table 7.
[0156]
[0157]
[0158]
Embodiment 2
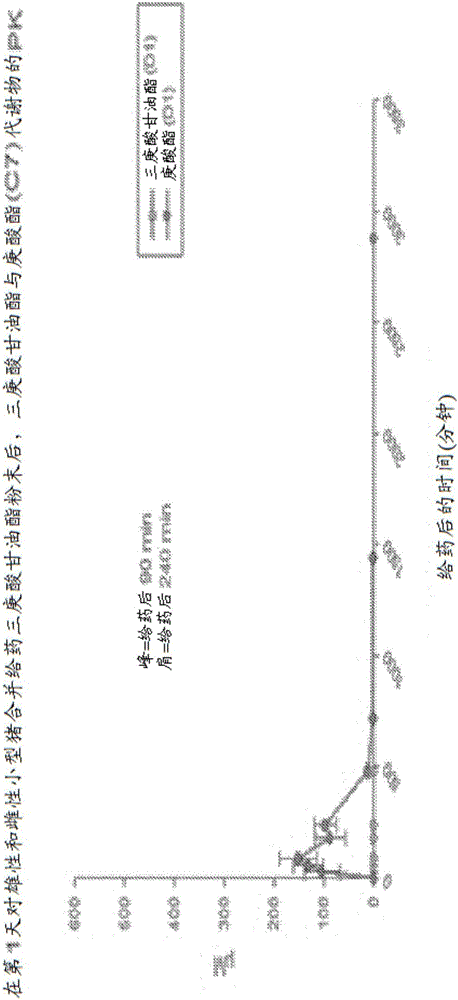
[0160] A single-dose study was performed for each group using a complete pharmacokinetic (PK) profile within 48 hours of dosing to determine when metabolites returned to baseline (with a one-week washout period between groups) and the relationship between gastrin and gallbladder Does the rapid release of the kinocin (CCK) hormone cause spasmodic contractions of the stomach. Blood samples were collected from each group at any time between 0 and 90 min plus this window if stomach pain was observed.
[0161]More specifically, animals (n=3 for each sex) were fasted before dosing and fed 4 hours after dosing. Blood samples were collected from pre-dose to 48 hours post-dose for PK analysis of triheptanoin and metabolites. As seen in the multiple dose study (Example 3), there were no major differences between males and females, so the data for all metabolites were combined.
[0162] exist Figures 1 to 21 , animals were administered a single oral gavage dose level of a triheptanoi...
Embodiment 3
[0175] A multiple dose study was performed on oil, powder and powder-ER as used in Example 2 and the results are shown below.
[0176] ·Powder group:
[0177] Day 1: Animal #6501 vomited approximately 1 hour after dosing
[0178] Day 3: Animal #6502 vomits
[0179] Day 3: Animal #6510 stomach pain observed
[0180] · Oil group:
[0181] Day 1: Animal #6501 vomited approximately 3 hours after dosing
[0182] Day 1: Animal #6516 vomited approximately 3 hours after dosing
[0183] Day 5: Stomach pain observed in animal #6509
[0184] ·Powder ER team:
[0185] No vomiting or stomach pain observed
[0186] enanthate
[0187] In conclusion, the results were as expected, triheptanoin released heptanoic acid. refer to Figure 22 , Triheptanoin is mainly metabolized in the liver into C7 fatty acids and ketone bodies, which are distributed to other tissues through circulation to provide energy sources. No triheptanoin values in the oil panel. All triheptanoin oils were con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com