Solid compositions of triglycerides and uses thereof
a technology of triglyceride and solid composition, which is applied in the field of solid composition of triglyceride, can solve the problems of gastrin-related stomach spasm and emesis, difficulty in handling, carrying, and dispense, and difficulty in administering liquid dosage forms,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0132]Table 5 shows the high purity of exemplary triheptanoin oil samples and solid samples comprising triheptanoin oil in accordance with the present invention.
[0133]Further, the stability of an exemplary solid composition (solid sample 2 in Table 5) comprising triheptanoin oil was tested. Measurements were conducted after storage at 25° C. / 60% RH (relative humidity) in double LDPE (low density polyethylene) bags in a 60 cc induction sealed HDPE (high density polyethylene) bottle and results are shown in Table 6. Measurements were also conducted after storage at 40° C. / 75% RH in double LDPE bags in a 60 cc induction sealed HDPE bottle and results are shown in Table 7.
TABLE 5Initial Purity Test ResultsParameterSpecificationOIL Sample 1OIL Sample 2SOLID Sample 1SOLID Sample 2SOLID Sample 3ImpuritiesGlycerol≤1.0%NDNDNDNDNDMonoheptanoate≤0.5%NDNDNDNDNDDiheptanoate≤1.5%1.2%0.7%1.1%1.1%1.1%Hexano-≤1.0%0.4%0.4%0.4%0.4%0.4%DiheptanoateIndividualUnidentified≤0.5%RRT 0.91: 0.06%RRT 1.26: 0.2...
example 2
[0134]Single dose study of each arm with full pharmacokinetics (PK) profile through 48 hours post dose was performed to determined when metabolites return to baseline (one week washout between each arm) as well as if acute release of gastrin and cholesystokinin (CCK) hormones cause spasmodic stomach contractions. Blood samples were collected from each arm 0-90 min plus anytime outside this window if gastric distress observed.
[0135]More specifically, animals (n=3 / sex) were fasted prior to dose and fed 4 hrs post dose. Blood samples for PK analysis of triheptanoin and metabolites were collected pre-dose through 48 hrs post dose. As seen in the multiple dose study (Example 3), there were no major differences between males and females thus data was combined for all metabolites.
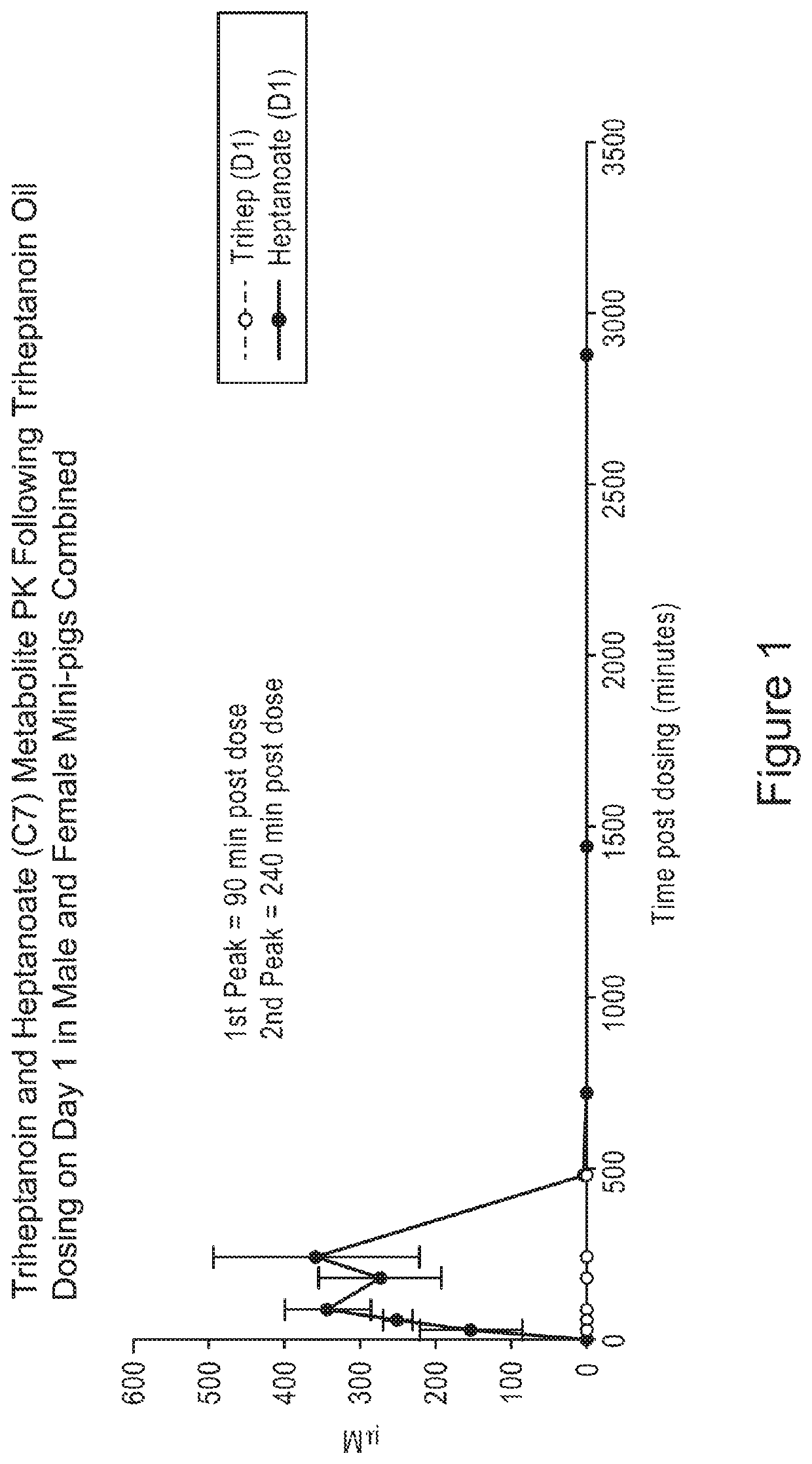
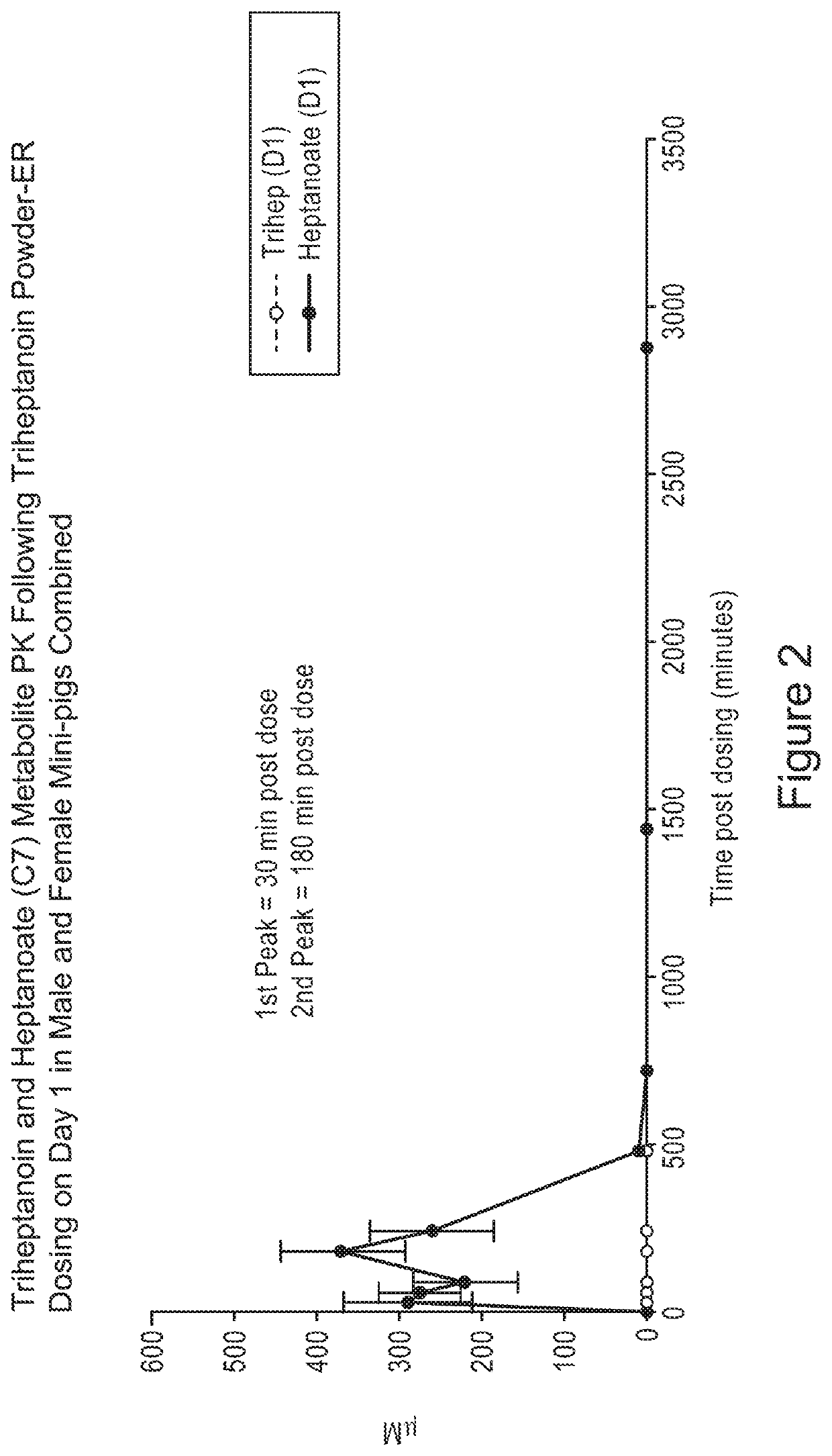
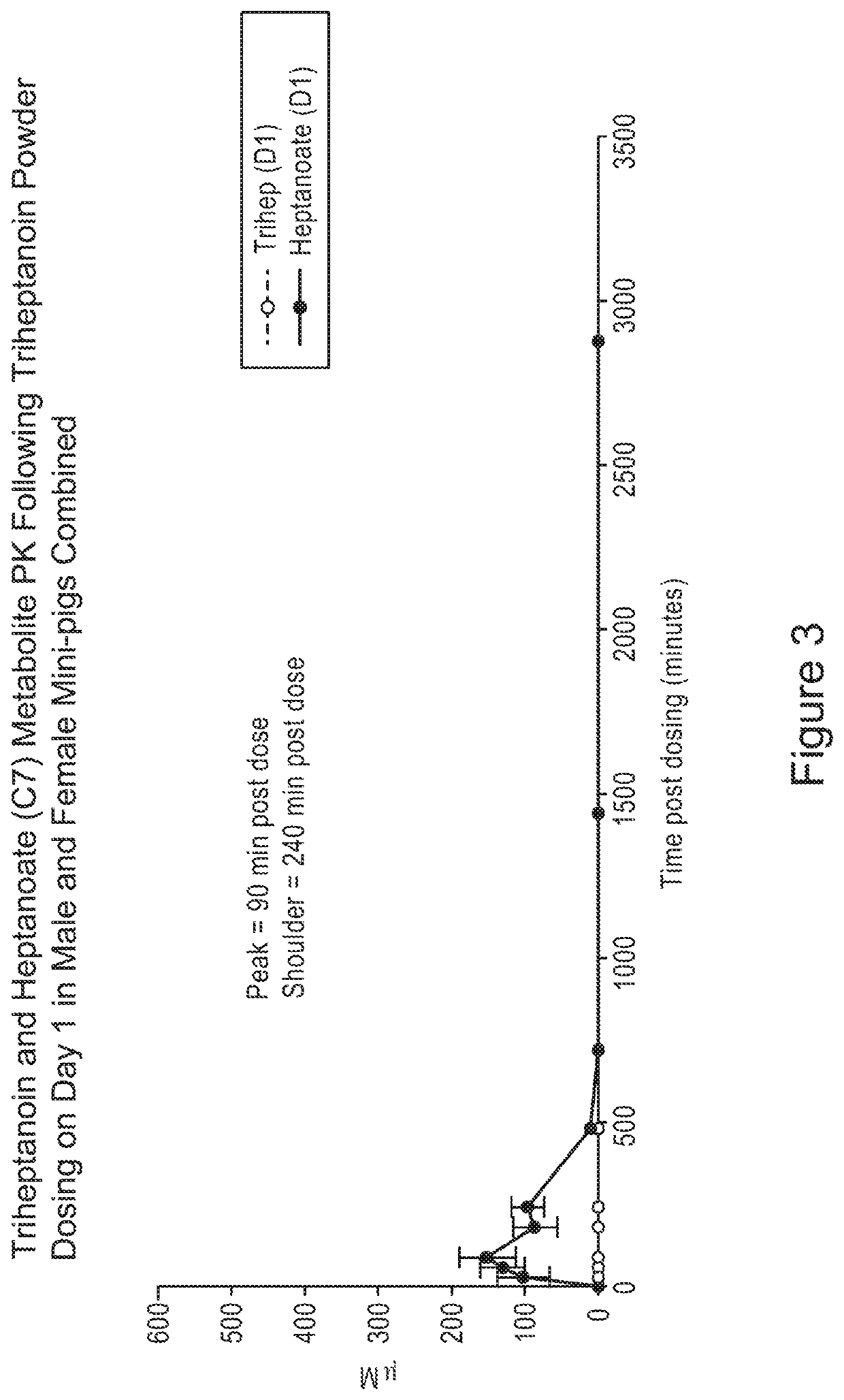
[0136]In FIGS. 1-21, animals were administered a single oral gavage dose level of a triheptanoin oil sample (i.e., the oil) or exemplary solid samples comprising triheptanoin oil in accordance with the present inv...
example 3
[0148]Multiple dose study was performed on the oil, the Powder and the Powder-ER as used in Example 2 and the results are shown below.
[0149]Powder Arm:[0150]Day 1: Animal #6501 vomited ˜1 hour post dose[0151]Day 3: Animal #6502 vomited[0152]Day 3: Animal #6510 gastric distressed observed
[0153]Oil Arm:[0154]Day 1: Animal 46501 vomited ˜3 hours post dose[0155]Day 1: Animal 46516 vomited ˜3 hours post dose[0156]Day 5: Animal #6509 gastric distressed observed
[0157]Powder ER Arm:[0158]No vomiting or gastric distress observed
[0159]Heptanoic Acid Across Dose Groups
[0160]Overall, results as expected, the triheptanoin releases heptanoic acid. Referring to FIG. 22, triheptanoin was metabolized primarily in liver to C7 fatty acids and ketone bodies which distribute via circulation to other tissues to provide an energy source. There were no triheptanoin values in oil arm. All triheptanoin oil was converted to C7.
[0161]Trace amounts of triheptanoin were observed in powder arm. The powder matrix ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com