A method for predicting responsiveness to a treatment with an EGFR inhibitor
An inhibitor and prognostic technology, applied in biochemical equipment and methods, microbial measurement/testing, drug combination, etc., can solve problems such as undisclosed cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
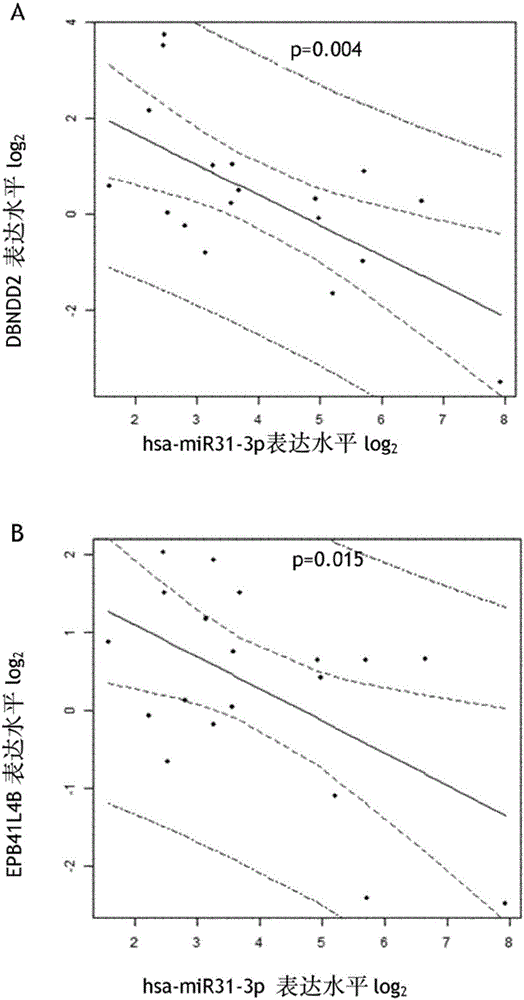
[0181] Example 1: DBNDD2 and EPB41L4B are targets of hsa-miR-31-3p and independently predict EGFR inhibitor response
[0182] patients and methods
[0183] patient
[0184] Twenty mCRC (metastatic colorectal cancer) patients constituted the patient group, 14 males and 6 females. The median age was 66.49±11.9 years old. All patients received the combination of irinotecan and cetuximab. Chemotherapy drugs recorded prior to the introduction of cetuximab belonged to the number of chemotherapy lines. The median follow-up until progression was 20 weeks, and the median overall survival was 10 months. All samples were from resections and fixed in formalin and paraffin embedded (FFPE).
[0185] Cell Culture and Transfection
[0186] We selected 3 colorectal adenocarcinoma cell lines weakly expressing hsa-miR-31-3p from the American Type Culture Collection (ATCC, Manassas, CA): HTB-37, CCL-222 and CCL-220 -1. HTB-37 was maintained in Dulbecco's Modified Eagle's Medium (DMEM)...
Embodiment 2
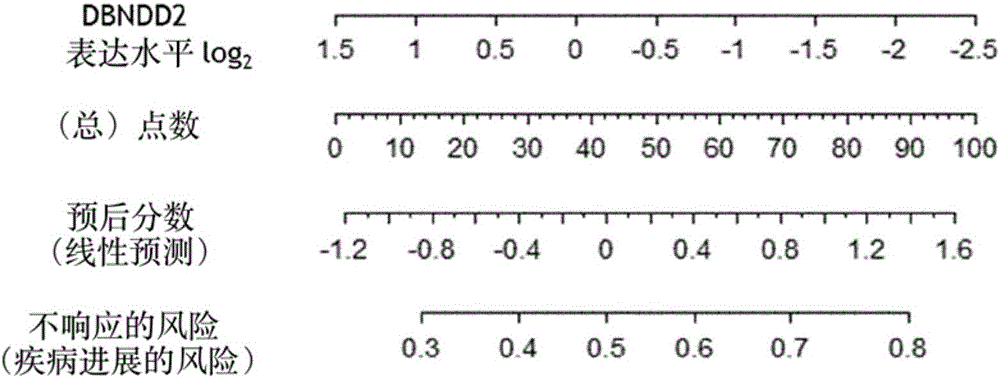
[0208] Example 2: Creation of a Tool Using DBNDD2 and EPB41L4B Expression to Predict Response to EGFR Inhibitors
[0209] patients and methods
[0210] patient
[0211] Twenty mCRC patients constituted the patient group (13 males, 7 females). The median age was 67±11.2 years old. All had metastatic disease at enrollment. All of these patients had KRAS wild-type metastatic colon cancer. All patients were considered refractory to 5-fluorouracil-based regimens in combination with irinotecan and oxaliplatin. They received anti-EGFR-based chemotherapy, panitumumab in 8 patients, cetuximab in 10 patients, and a combination of panitumumab and cetuximab in 2 patients. Chemotherapy drugs recorded before the introduction of cetuximab and panitumumab belonged to the few-line drugs. The median follow-up until progression was 21 weeks, and the median overall survival was 8.9 months.
[0212] Measurement of Gene Expression
[0213] qRT-PCR for the expression of DBNDD2 and EPB41L...
Embodiment 3
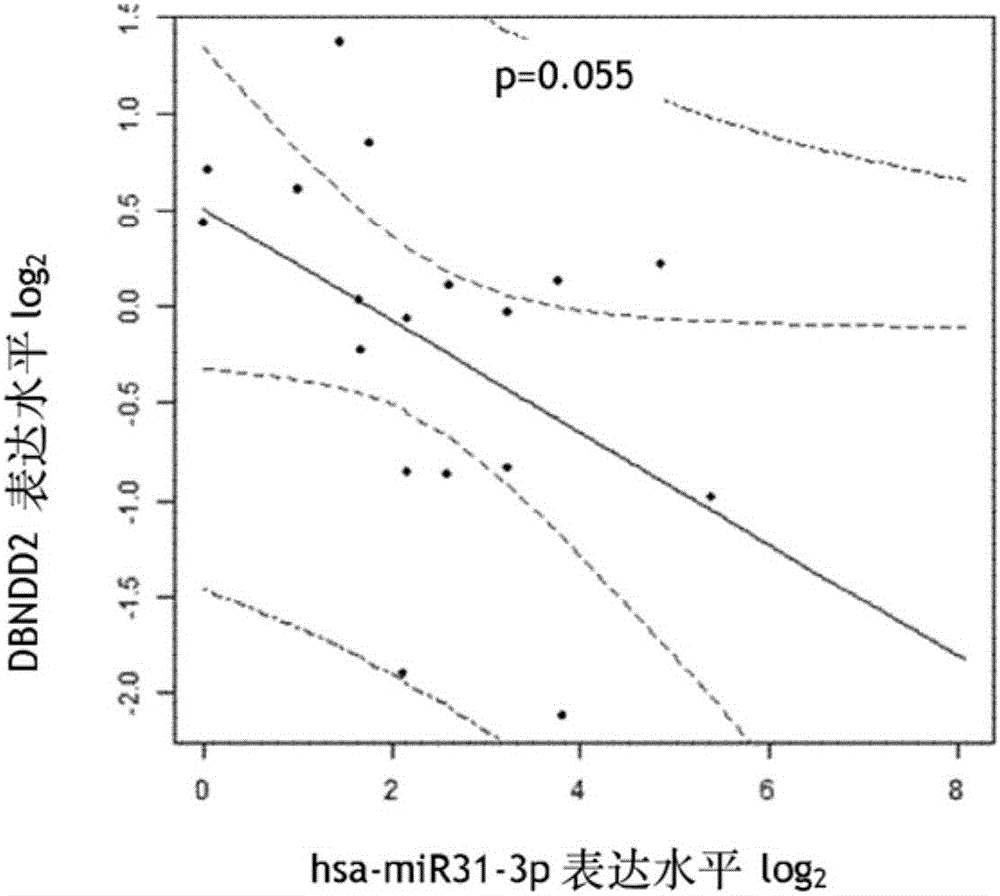
[0220] Example 3: Replication of the predictive value of DBNDD2 and EPB41L4B for EGFR inhibitors in a new and independent population
[0221] patients and methods
[0222] patient
[0223] Forty-two mCRC (metastatic colorectal cancer) patients constituted the patient group, 27 males and 15 females. The median age was 59±12.1 years old. All had metastatic disease at enrollment. Based on the protocol, all patients were treated with third-line therapy with irinotecan and panitumumab after progression on chemotherapy with oxaliplatin and irinotecan. The median follow-up until progression was 23 weeks, and the median overall survival was 9.6 months. Twenty-six samples were provided as FFPE and 16 as frozen tissue.
[0224] Measurement of Gene Expression
[0225] qRT-PCR validation of target expression from frozen or FFPE patient samples was performed with 20 ng of total RNA using the ABI7900HT Real-Time PCR System (Applied Biosystem). All reactions were performed in trip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com