A kind of aluminum electrolysis method using aluminum as cathode
A technology for aluminum electrolysis and aluminum electrolysis cell, which is applied in the field of improvement of aluminum electrolysis production method by molten salt method, can solve the problems of less than 36% of aluminum electrolysis energy utilization efficiency, increased cathode furnace bottom pressure drop, and reduced aluminum electrolysis current efficiency, etc. Achieve the effect of eliminating defects of honeycomb micro-battery and eliminating alumina precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
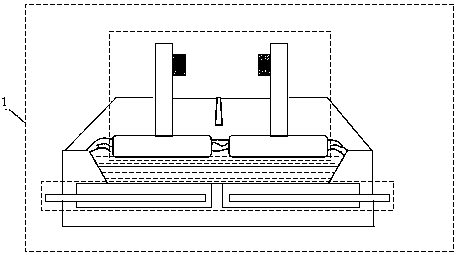
[0028] An aluminum electrolysis method using aluminum as the cathode, the electrolysis process is to use the aluminum liquid at the bottom of the electrolytic cell 4 as the cathode for aluminum electrolysis; the aluminum liquid 6 at the bottom of the electrolytic cell contacts with the cathode bus 5 to form an aluminum electrolysis process cathode.
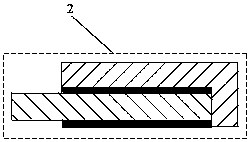
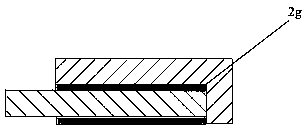
[0029] In the aluminum electrolysis method using aluminum as the cathode of the present invention, a partition wall 7 is fixedly connected to the side wall of the electrolytic cell on the inner side of both ends of the aluminum electrolytic cell body, and the bottom of the partition wall extends into the cathode aluminum liquid, and is connected with the electrolytic cell side wall. The bottom of the electrolytic cell forms the lower channel of the aluminum liquid, the partition wall and the inner walls of the two ends of the aluminum electrolytic cell form the side channel of the aluminum liquid, and the cathode busbar 5 contacts ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com