Aluminum electrolysis cell cathode as which aluminum is adopted
An aluminum electrolytic cell and electrolytic cell technology are applied in the field of aluminum electrolytic cell cathodes with aluminum as the cathode, which can solve the problems of insufficient energy utilization efficiency of aluminum electrolysis, increase power consumption of aluminum electrolysis, increase power consumption of electrolytic aluminum, and the like. The effect of eliminating alumina precipitation and defects in honeycomb microbatteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
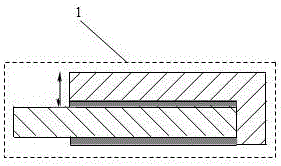
[0020] The cathode of an aluminum electrolytic cell with aluminum as the cathode, the structure of the cathode includes:
[0021] An electrolytic cell body 2, the electrolytic cell body is a concave cell body made of refractory-insulation material; a groove side 3a is provided on the upper edge of the electrolytic cell body;
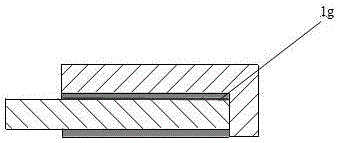
[0022] The cathode partition wall is composed of a side refractory-insulation layer 2g and a side carbon block 2f. A partition wall, a bottom channel 2k is provided between the bottom of the partition wall and the lower bottom of the aluminum electrolytic cell body; the cathode partition wall and the inner wall-shaped side channel at the end of the aluminum electrolytic cell body;
[0023] The cathode busbar 2h, the cathode busbar enters the electrolytic cell body through the bottom channel 2k at the bottom of the partition wall.
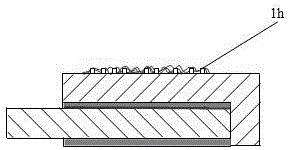
[0024] In the aluminum electrolytic cell cathode using aluminum as the negative electrode of the present invention, its cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com