Medical device administration event report and safety early warning information orientation transmission system
A technology for adverse events and medical devices, applied in transmission systems, instruments, electrical components, etc., to improve accuracy and value, facilitate investigation, and improve utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
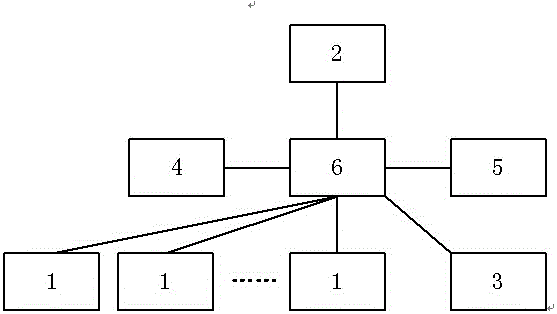
[0025] like figure 1 As shown, the medical device adverse event reporting and safety warning information directional transmission system of the present invention includes: mobile reporting terminal 1 , reporting unit 2 , approval terminal 3 , database 4 , information processing unit 5 and server 6 . The mobile reporting terminal 1, the reporting unit 2, the approval terminal 3, the database 4 and the information processing unit 5 are all connected to the server 6 through a network (wired or wireless).
[0026] The server 6 can be realized by a computer meeting the function and performance requirements of the system. The server 6 is respectively connected with the mobile reporting terminal 1, the reporting unit 2, the approval terminal 3, the database 4 and the information processing unit 5 through a wired or wireless network, and can realize mutual network communication among them. In order to ensure the mobility of the mobile reporting terminal 1, the server 6 and the mobile...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com