Glaucocalyxin A-carrying bilirubin albumin nanoparticles and preparation method and application thereof
A technology of albumin nanoparticles and cyanine A, which is applied in the direction of non-active ingredients of polymer compounds, powder delivery, drug combination, etc., to achieve the effect of improving poor water solubility and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Organic phase preparation: disperse the cerulein A drug (13.33%) in the organic phase;
[0053] Water phase preparation; adjust the pH to 4.0 with deionized water, add albumin (1%) to fully dissolve;
[0054] Slowly inject the organic phase into the water phase while dispersing at high shear (32000rpm, 5min) to obtain colostrum;
[0055] The colostrum was subjected to high-pressure homogenization (8000psi, 8 times) to obtain uniformly dispersed cyanine-loaded albumin nanoparticles;
[0056] The obtained cyanine-loaded albumin nanoparticles were vacuum rotary evaporated at 40° C. for 60 minutes to remove the organic phase; the cyanine-loaded albumin nanoparticles were filtered through a 0.22 μm carbonate sterile filter.
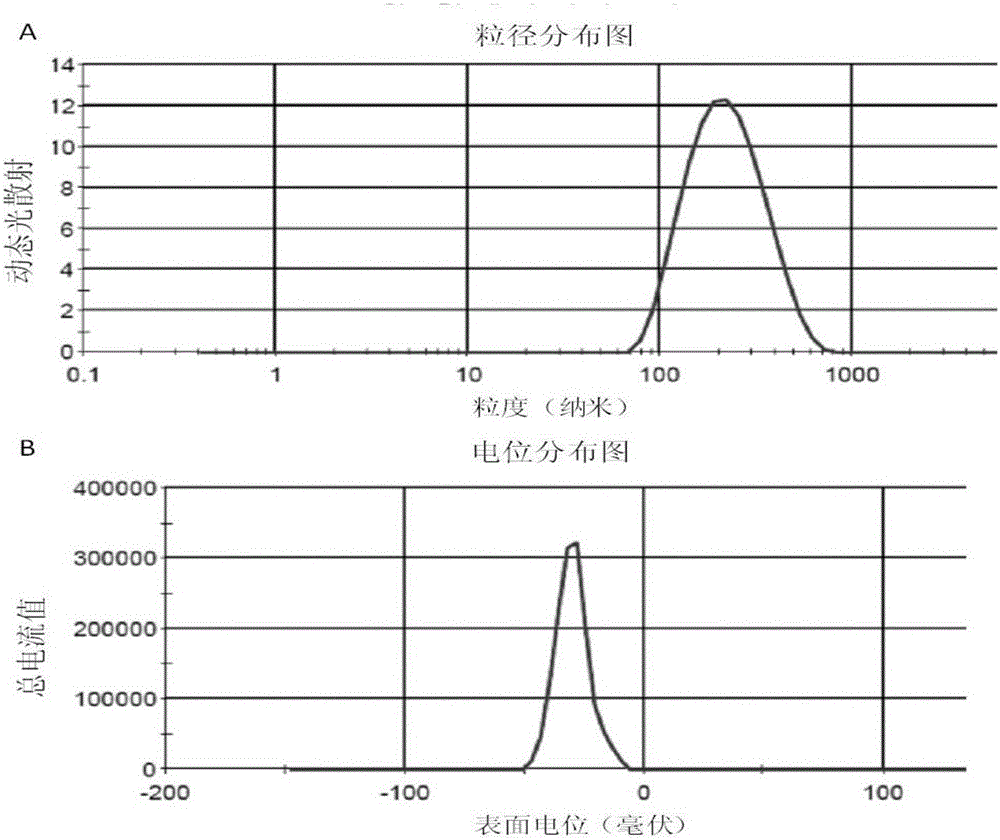
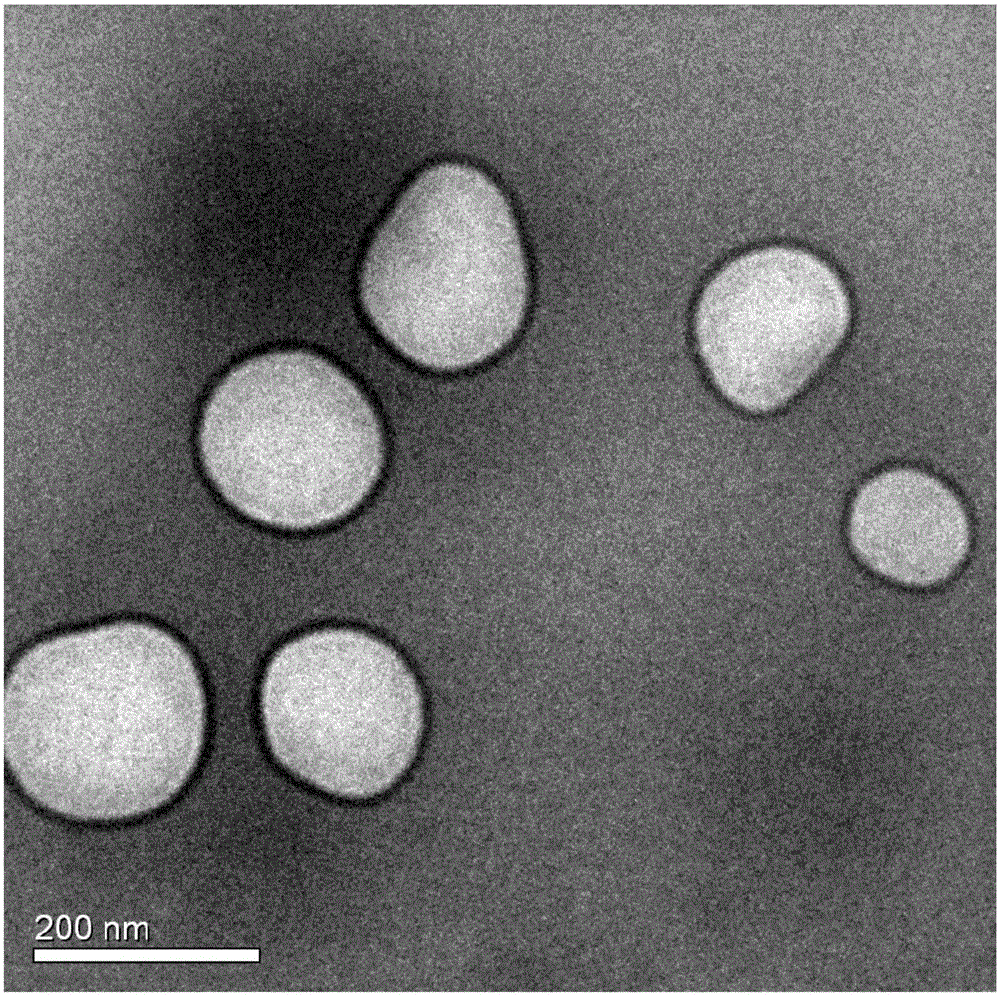
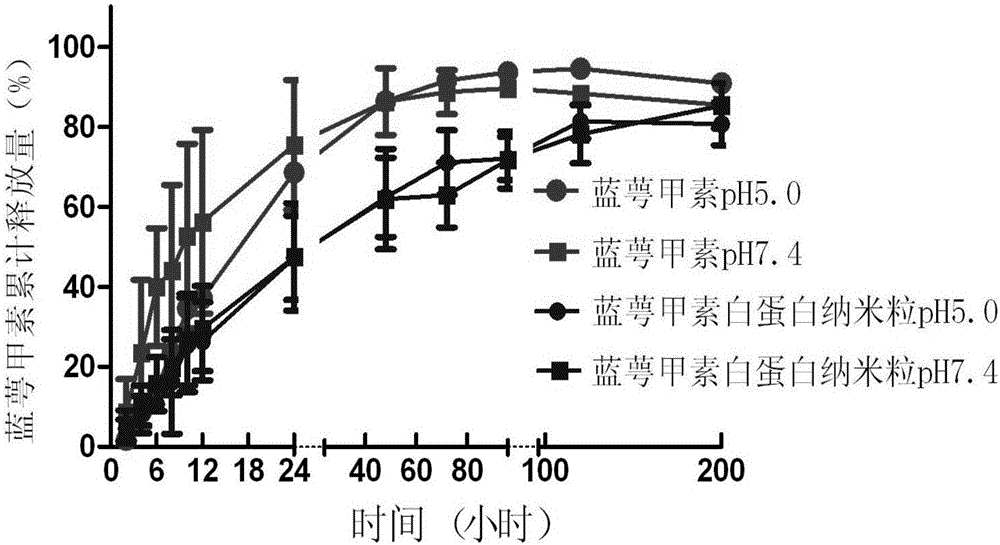
[0057] The nanoparticle suspension was measured by a Malvern particle size analyzer, and its average particle diameter was 84.77±2.050nm, its Zeta potential was -17.73±3.772, its encapsulation efficiency was 22.69%, and its stability was less than 48h. ...
Embodiment 2
[0059] Organic phase preparation: disperse the cerulein A drug (26.66%) in the organic phase;
[0060] Water phase preparation; adjust the pH to 4.0 with deionized water, add albumin (1%) to fully dissolve;
[0061] Slowly inject the organic phase into the water phase while dispersing at high shear (32000rpm, 5min) to obtain colostrum;
[0062] The colostrum was subjected to high-pressure homogenization (16000psi, 16 times) to obtain uniformly dispersed scutellarin-loaded albumin nanoparticles;
[0063] The obtained cyanine-loaded albumin nanoparticles were vacuum rotary evaporated at 40° C. for 60 minutes to remove the organic phase; the cyanine-loaded albumin nanoparticles were filtered through a 0.22 μm carbonate sterile filter.
[0064] The nanoparticle suspension was measured by a Malvern particle size analyzer, and its average particle diameter was 121.07±31.13nm, its Zeta potential was -21.20±2.514, its encapsulation efficiency was 39.68%, and its stability was less th...
Embodiment 3
[0066] Organic phase preparation: disperse the cerulein A drug (40%) in the organic phase;
[0067] Water phase preparation; adjust the pH to 4.0 with deionized water, add albumin (1%) to fully dissolve;
[0068] Slowly inject the organic phase into the water phase while dispersing at high shear (32000rpm, 5min) to obtain colostrum;
[0069] The colostrum was subjected to high-pressure homogenization (24000psi, 24 times) to obtain uniformly dispersed scutellarin-loaded albumin nanoparticles;
[0070] The obtained cyanine-loaded albumin nanoparticles were vacuum rotary evaporated at 40° C. for 60 minutes to remove the organic phase; the cyanine-loaded albumin nanoparticles were filtered through a 0.22 μm carbonate sterile filter.
[0071] The nanoparticle suspension was measured by a Malvern particle size analyzer, and its average particle diameter was 191.77±19.25nm, its Zeta potential was -20.1±0.860, its encapsulation efficiency was 53.23%, and its stability was less than 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com