Treatment of stroke using isolated placental cells
A cell and placenta technology, applied in the field of stroke treatment using isolated placental cells, can solve the problem of no effective treatment for stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0225] 5.7 Preparation of placental cell bank
[0226] Isolated cells from postpartum placenta, e.g., those described in Section 5.4.2 above, can be cultured in a number of different ways to prepare a set of batches, e.g., wherein each batch is a plurality of individually administrable doses . Such a batch can be obtained, for example, from cells from placental perfusate or from cells from enzymatically digested placental tissue. A batch of placental cells obtained from a plurality of placentas can be arranged in a separate placental cell bank, eg, for long-term storage. Typically, placental cells in plastic-adherent tissue culture are obtained from primary cultures of placental material to form seed cultures, which are expanded under controlled conditions to form approximately doublings of cell populations. A batch is preferably derived from a single placental tissue, but can be derived from multiple placental tissues.
[0227] In one embodiment, a placental cell batch is ...
Embodiment 1
[0297] 6.1 Example 1: Intracranial Administration of Isolated Placental Cells for Stroke
[0298] This example demonstrates the efficacy of administering isolated placental cells in the treatment of conditions associated with disruption of blood flow in or around the brain or CNS.
[0299] CD34 is obtained by enzymatic digestion with about 1 to about 2 mg / ml of collagenase I, e.g., for 30 minutes, followed by enzymatic digestion with about 0.25% trypsin at 37° C. for 10 minutes. - , CD10 + , CD105 + 、CD200 + Placental cells in plastic-adherent tissue culture. Sprague-Dawley rats were used as a stroke model. The rat is an established model animal used to study the outcome of stroke and the effect of different therapies on stroke symptoms. See, eg, Chen et al., "AModel of Focal Ischemic Stroke in the Rat: Reproducible Extensive Cortical Infarction", Stroke 17(4):738-743 (1986). Ten animals were used for each condition.
[0300] MCA stroke surgery: All surgical procedures ...
Embodiment 2
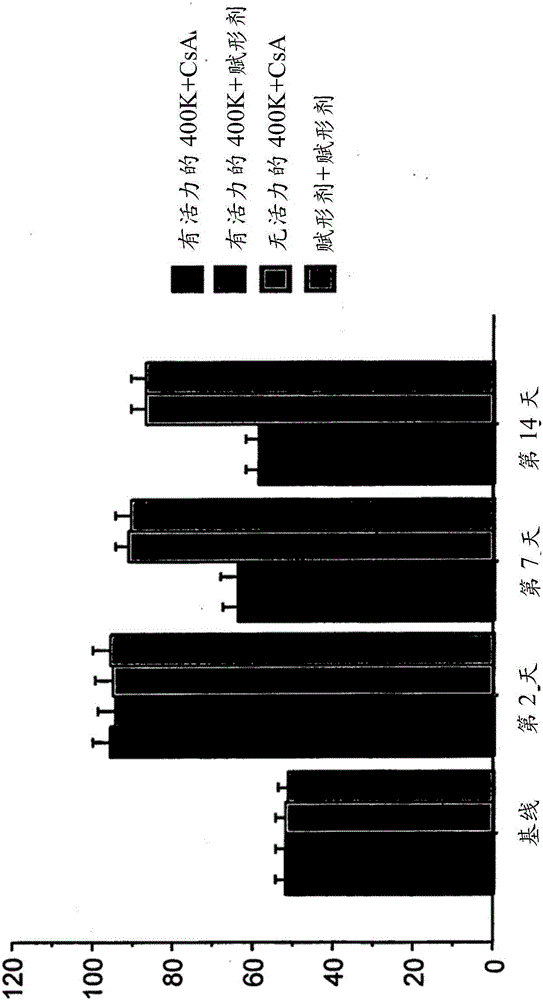
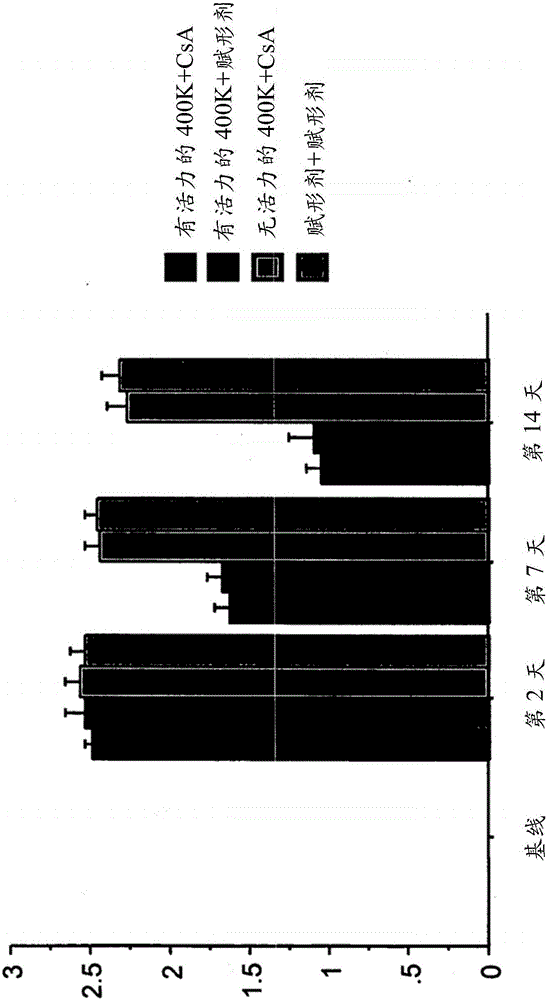
[0310] 6.2 Example 2: Treatment of Stroke Using Intravenously Administered Isolated Placental Cells
[0311] This example demonstrates the efficacy of intravenous administration of isolated placental cells in the treatment of conditions associated with disruption of blood flow in or around the brain or CNS, such as the treatment of hypoxic or hypoxic injury. For example, the results presented here demonstrate that intravenous administration of viable human placental cells promotes dose-dependent behavioral recovery in motor and neurological assays in animals administered placental cells compared to animals receiving non-viable human placental cells .
[0312] Isolated CD34 was obtained by enzymatic digestion as described elsewhere herein - , CD10 + , CD105 + 、CD200 + Placental cells in plastic-adherent tissue culture. Sprague-Dawley rats were used as a stroke model, and the middle arteries were occluded as described in Example 1 above. On day 2 after blocking, recipient ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com