A kind of water-soluble pegylated fischer carbene compound and preparation method thereof
A technology for PEGylation and compounding, which is applied in the field of water-soluble PEGylation Fischer carbene compounds and their preparation, can solve problems such as difficult derivatization of ligands, achieve simple synthesis methods, reduce toxicity, and increase water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
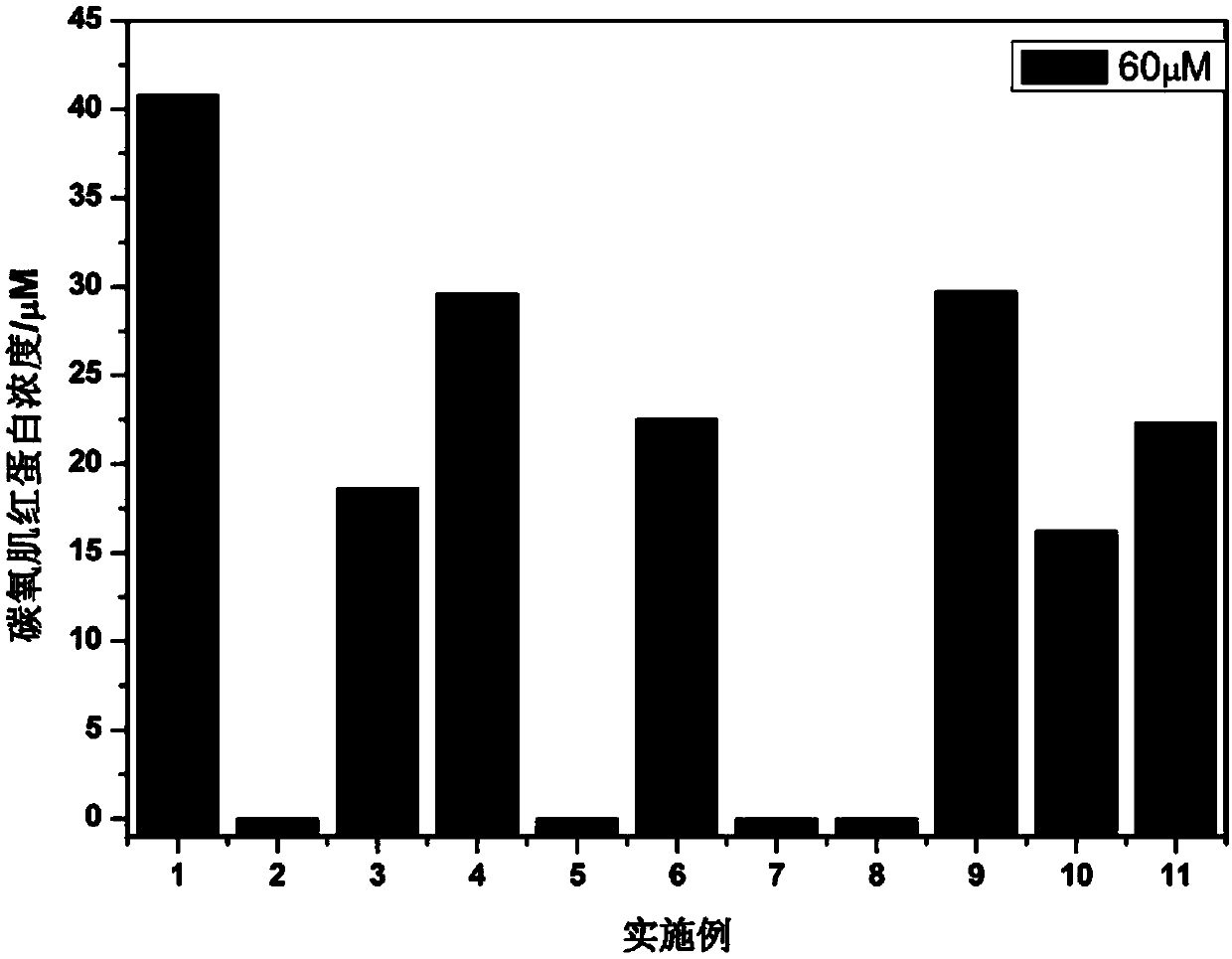
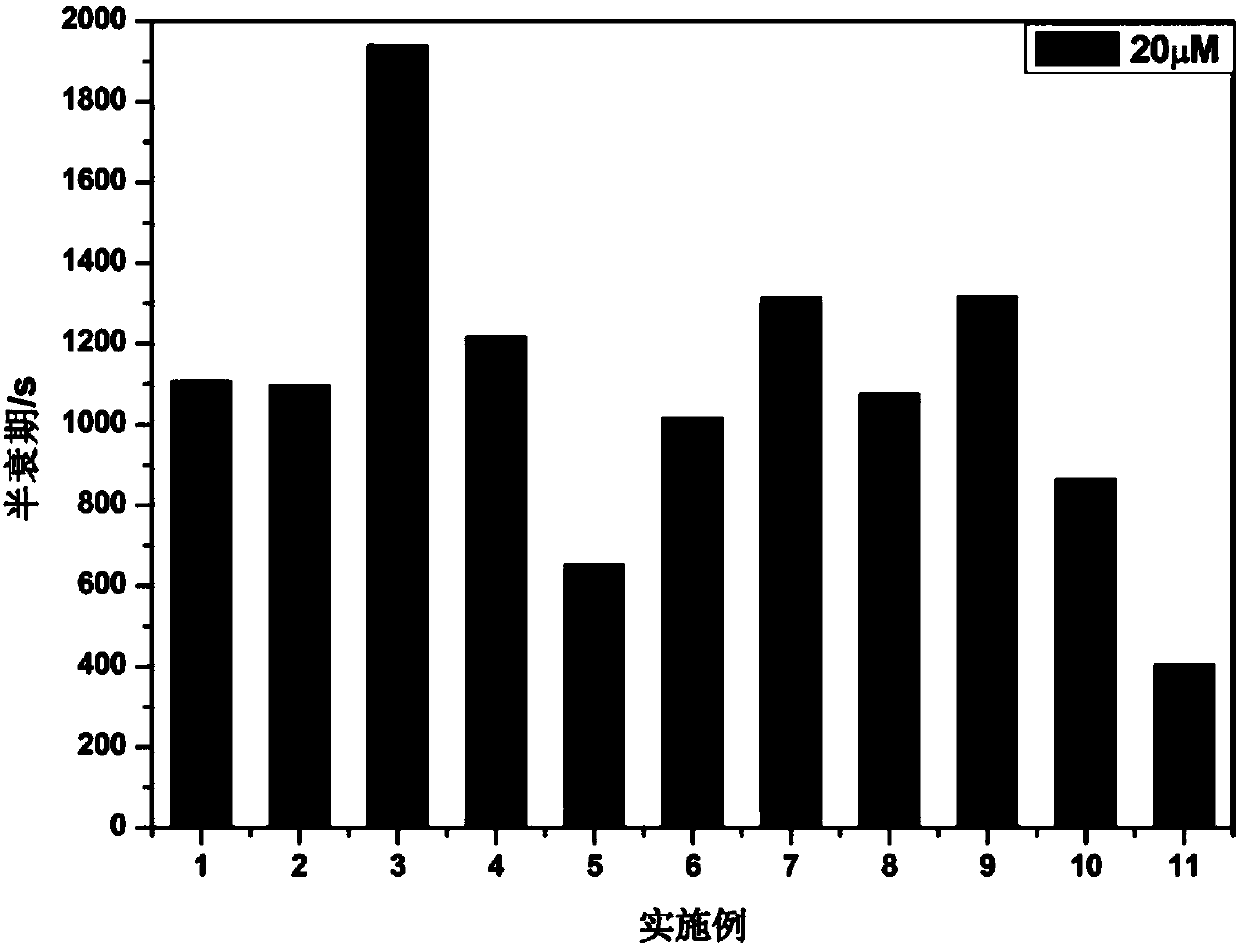
Examples
Embodiment 1
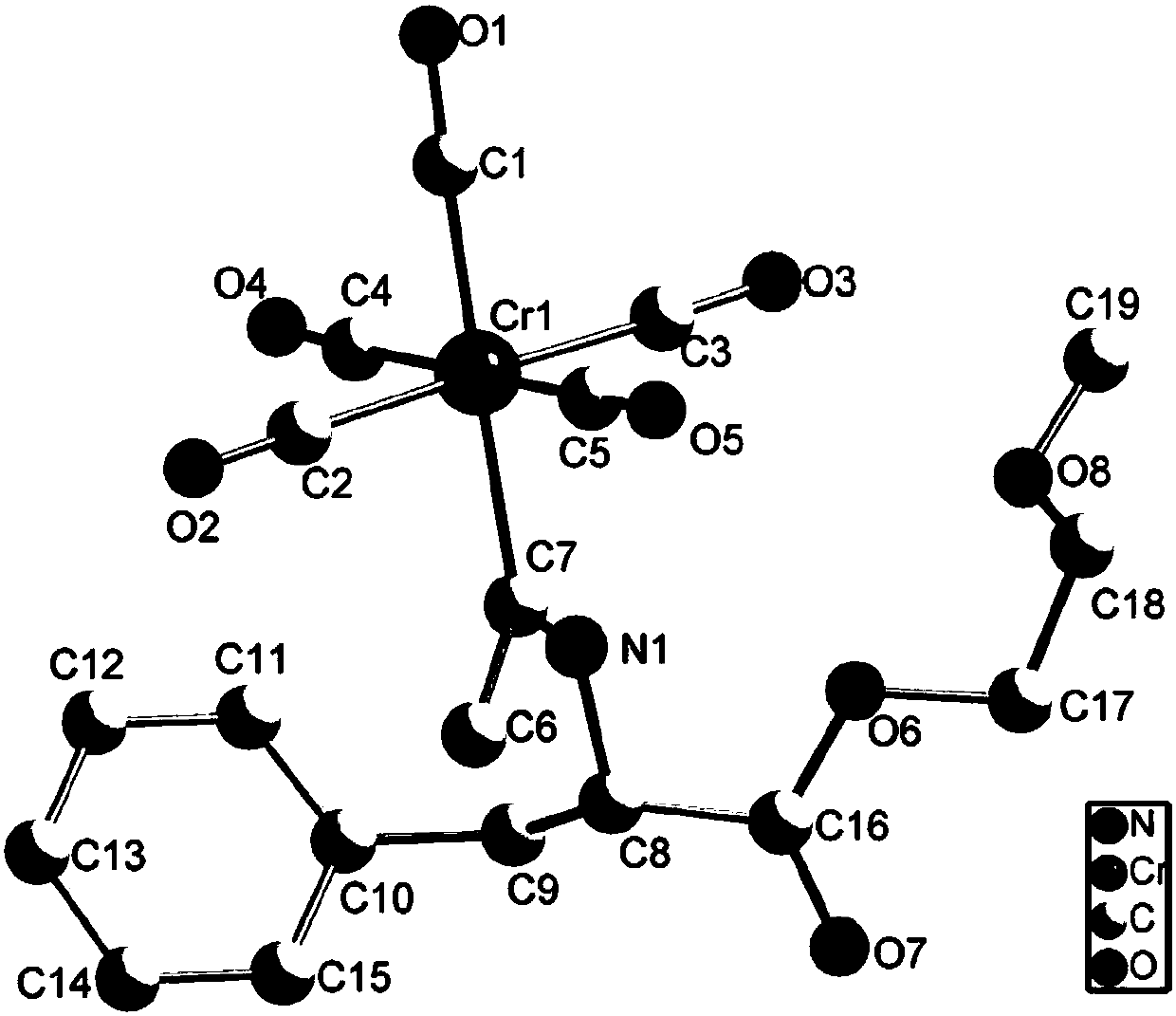
[0026] Take the preparation of the following PEGylated Fischer carbene compound as an example:
[0027]
[0028] Take by weighing 500.2mg (1mmol) Fischer carbene of chromium pentacarbonyl shown in formula 1-1 and 265.6mg (1mmol) monoethylene glycol monomethyl ether amino acid ester hydrochloride and place in Schlenk bottle, adopt vacuum line operation technique, In the absence of water and oxygen, N 2 (99.999%) protection, add 15mL of dry methanol and 140 μL (1mmol) triethylamine, stir at room temperature for 5 hours, column chromatography (with the volume ratio of dichloromethane and petroleum ether being 3:1 mixed solution as the elution liquid), to obtain oily yellow PEGylated Fischer carbene compound (112.9mg, yield 64%), its reaction equation is as follows:
[0029]
[0030] The structural characterization data of the resulting product is: IR(CH 2 Cl 2 , cm -1 ): Vco=2056cm -1 , 1928cm -1 , 1748cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ(ppm): 9.34(s, 1H), 9.16-9.04...
Embodiment 2
[0032] Take the preparation of the following PEGylated Fischer carbene compound as an example:
[0033]
[0034] In Example 1, the monoethylene glycol monomethyl ether glycinate hydrochloride used is replaced with equimolar monoethylene glycol monomethyl ether phenylalanine hydrochloride, and other steps are the same as in Example 1 to obtain Yellow oily PEGylated Fischer carbene compound (276mg, yield 62%), its reaction equation is as follows:
[0035]
[0036] The structural characterization data of the resulting product is: IR(CH 2 Cl 2 , cm -1 ): Vco=2056cm -1 , 1927cm -1 , 1744cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 9.16 (s, 1H), 8.75 (s, 0.35H), 7.33 (s, 3H), 7.19 (s, 2H), 4.77 (s, 1H), 4.46-4.28 (m, 2H) , 3.58(d, 2H), 3.40(s, 3H), 3.17(d, 2H), 2.29(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ (ppm): 282.38, 224.13, 218.82, 170.46, 135.12, 130.83, 129.44, 71.27, 66.72, 61.62, 60.32, 40.60, 36.95.
[0037] The obtained product was dissolved in dichloromethane, a...
Embodiment 3
[0039] Take the preparation of the following PEGylated Fischer carbene compound as an example:
[0040]
[0041] In Example 1, the monoethylene glycol monomethyl ether glycinate hydrochloride used is replaced with equimolar monoethylene glycol monomethyl ether tyrosine hydrochloride, and other steps are the same as in Example 1 to obtain yellow Oily PEGylated Fischer carbene compound (103mg, yield 23%), its reaction equation is as follows:
[0042]
[0043] The structural characterization data of the resulting product is: IR(CH 2 Cl 2 , cm -1 ): Vco=2056cm -1 , 1928cm -1 , 1735cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 9.14 (s, 1H), 7.02 (s, 2H), 6.82 (s, 2H), 6.51-5.74 (m, 2H), 4.70 (s, 1H), 4.09 (s, 2H), 3.86(s, 3H), 3.08(d, 2H), 2.32(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ (ppm): 285.04, 222.78, 217.44, 171.61, 155.82, 130.66, 125.76, 116.00, 69.87, 65.15, 60.49, 58.90, 38.23, 35.57.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com