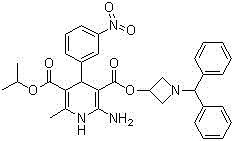
Method for separating chiral drug azelnidipine enantiomer through simulated moving bed chromatography technique
A technology of simulating moving bed and azedipine, which is applied in the field of separation of chiral drugs, can solve the problems of unsatisfactory large-scale industrial production and small preparation volume, and achieve the effect of low cost, less pollution and continuous production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a. Operating conditions
[0036] Injection concentration: racemate Azeldipine concentration 20g / L
[0037] Injection liquid flow rate: U F =3.1ml / min
[0038] Eluent flow rate: U D =12.0ml / min raffinate flow rate: U R =6.9ml / min
[0039] Extraction flow rate: U E =5.8ml / min
[0040] Switching time: t s =1.5min
[0041] b. Separation step
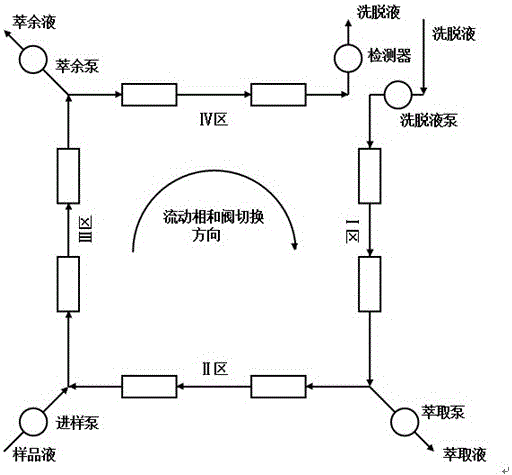
[0042](1) Fully dissolve the racemate Azedipine in the prepared mobile phase, the concentration is 20g / L, as the feed liquid, the sample solution and the eluent are sequentially injected into the chromatographic system from the sample inlet and the eluent inlet , by simulating the controller of the moving bed chromatography system, the opening and closing of the pneumatic valve is regularly controlled, so that the eluting port, sample inlet, extract and raffinate outlets are regularly changed along the direction of the mobile phase, and the switching time is 1.5s. The two single enantiomers of Azedipine flow out of the syste...
Embodiment 2
[0049] a. Operating conditions
[0050] Injection concentration: racemate Azeldipine concentration 40g / L
[0051] Injection liquid flow rate: U F =2.4ml / min
[0052] Eluent flow rate: U D =18.0ml / min raffinate flow rate: U R =8.7ml / min
[0053] Extraction flow rate: U E =7.1ml / min
[0054] Switching time: t s =0.9min
[0055] b. Separation step
[0056] (1) Fully dissolve the racemate Azedipine in the prepared mobile phase, the concentration is 40g / L, as the feed liquid, the sample solution and the eluent are sequentially injected into the chromatographic system from the sample inlet and the eluent inlet , by simulating the controller of the moving bed chromatography system, the opening and closing of the pneumatic valve is regularly controlled, so that the eluting port, sample inlet, extract and raffinate outlets are regularly changed along the direction of the mobile phase, and the switching time is 0.9s, so that The two single enantiomers of Azedipine flow out of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com