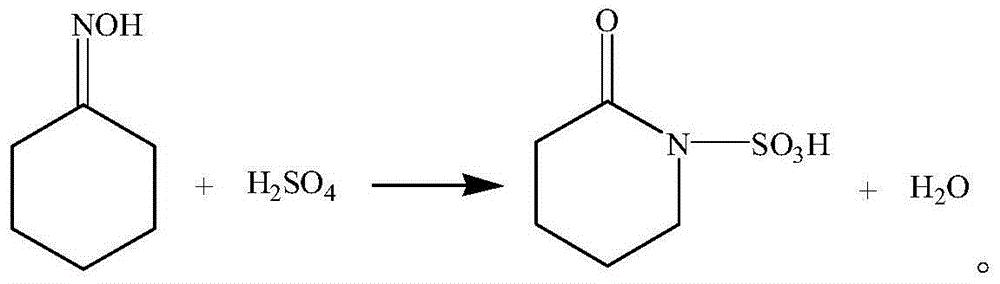
Preparation method for caprolactam
A technology of caprolactam and cyclohexanone, applied in the field of preparation of caprolactam, can solve the problems of low caprolactam yield, high ammonium sulfate by-product, long process flow, etc. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of caprolactam provided by the invention comprises the following steps:
[0018] (1) In the presence of an oximation catalyst and without any organic solvent, cyclohexanone, ammonia and hydrogen peroxide are carried out in an aqueous solution for ammoximation reaction, and the temperature of the ammoximation reaction is 50 -200°C, a solution containing cyclohexanone oxime was obtained;
[0019] (2) Extracting the solution containing cyclohexanone oxime obtained in step (1) with an inert organic solvent to obtain an extract phase containing cyclohexanone oxime and a raffinate phase containing an oximation catalyst and water;
[0020] (3) performing a Beckmann rearrangement reaction on the extract phase with oleum, and then neutralizing the product of the Beckmann rearrangement reaction with ammonia.
[0021] The present invention has no special limitation on the amount of water used in the ammoximation reaction system, and it can be reasonably add...
Embodiment 1
[0043] This example is used to illustrate the preparation method of caprolactam provided by the present invention.
[0044] In the ammoximation reactor, load 100t oximation catalyst aqueous solution, wherein, the content of oximation catalyst is 3% by weight, and then ammonia gas, cyclohexanone, hydrogen peroxide (concentration is 27.5% by weight) are respectively mixed with 1.94t / h , 10.9t / h and 15.2t / h flows into the ammoximation reactor for the ammoximation reaction, the controlled reaction temperature is 90-100°C, the reaction pressure is 300kPa (absolute pressure), and the material is The residence time in the reactor was 70 minutes, and an aqueous solution of cyclohexanone oxime was obtained. Hexanaphthene enters the cyclohexanone oxime aqueous solution (relative to the cyclohexanone of 1mol, the consumption of hexamethylene is 3mol) that the flow of 33t / h and the flow from the ammoximation reactor are 88t / h fully mixed and enters cyclohexane. liquid separator. The hea...
Embodiment 2
[0046] This example is used to illustrate the preparation method of caprolactam provided by the present invention.
[0047] In the ammoximation reactor, 100t aqueous solution of oximation catalyst is charged, wherein the content of oximation catalyst is 3% by weight, then ammonia, cyclohexanone, hydrogen peroxide (concentration is 27.5% by weight) are respectively mixed with 5.19t / h , 27.22t / h and 38.46t / h flows into the ammoximation reactor for the ammoximation reaction, the controlled reaction temperature is 80-85°C, the reaction pressure is 250kPa (absolute pressure), and the material is The residence time in the reactor was 80 minutes, and an aqueous solution of cyclohexanone oxime was obtained. Hexanaphthene enters the cyclohexanone oxime aqueous solution (relative to the cyclohexanone of 1mol, the consumption of hexamethylene is 3mol) that the flow of 82t / h comes from the flow of ammoximation reactor is 136t / h fully mixes and enters cyclohexane liquid separator. The he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com