Preparation and application of a fluorescent probe for rapid detection of cysteine
A cysteine and fluorescent probe technology, applied in the field of fluorescent probes, can solve the problems of unreachable, low detection limit, complex preparation method, etc., and achieve the effects of fast response speed, low detection limit and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the synthesis of fluorescent probe molecule
[0041] (a) Under nitrogen protection, dissolve 3.1 g (16 mmol) of 4-cyanobiphenol in 50 ml of acetonitrile, and add 2.8 g (24 mmol) of anhydrous MgCl 2 and 8.2 ml (60 mmol) of anhydrous triethylamine, and finally 6.1 g (220 mmol) of dry paraformaldehyde was added and reacted at 70°C for 8 hours. After the reaction was completed, the solution was acidified with a large amount of dilute hydrochloric acid, extracted with dichloromethane, and separated on a silica gel column to obtain 1.8 g of white solid product II with a yield of 52%.
[0042] (b) Under the protection of nitrogen, take 1.0 g (4.48 mmol) of compound II and place it in a 100 ml three-neck flask, add 30 ml of dry dichloromethane into it, and stir until it is completely dissolved. Then 7.5 ml (0.11 mol) of pyrrole and a catalytic amount of trifluoroacetic acid were added, and the reaction was carried out at room temperature for one hour. After the ...
Embodiment 2
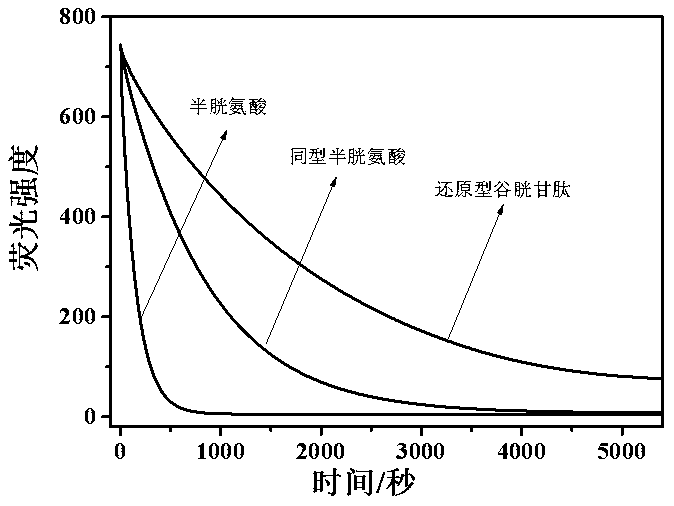
[0045] Example 2. Fluorescence response experiments of probe molecules to different thiol amino acids
[0046] Take the fluorescent probe compound of the present invention, dissolve it in acetonitrile, and prepare the probe molecule mother solution with a concentration of 50 μM; get a certain amount of cysteine, homocysteine and glutathione, and dissolve them in 100ml super Pure water, prepared to 10 -2 Different amino acid mother liquors with M concentration; take 2.38g of 4-hydroxyethylpiperazineethanesulfonic acid, dissolve it in 100 ml of ultrapure water, adjust the pH to 7.4 with sodium hydroxide, and prepare a buffer solution with a buffer concentration of 0.1 M.
[0047] Pipette 1ml probe molecule mother solution, add 4 ml acetonitrile, 1 ml HEPES buffer solution, 3.7 ml ultrapure water to it, add 0.3 ml Cys, Hcy and GSH mother solution to it respectively. Immediately start scanning its fluorescence intensity over time. Such as figure 2 , the results show that di...
Embodiment 3
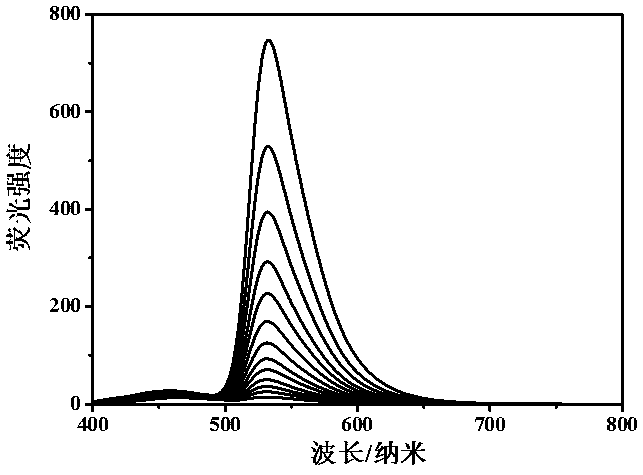
[0048] Example 3. Fluorescence response experiment of probe molecules to cysteine at different concentrations
[0049] Pipette 13 groups of 1 ml probe molecule mother solution, add 4 ml acetonitrile, 1 ml HEPES buffer solution, add 3.7 ml, 3.725 ml, 3.75 ml, 3.775ml, 3.80ml, 3.825ml, 3.85ml, 3.875ml, 3.90ml, 3.925ml, 3.950ml, 3.975ml, 4.0 ml ultrapure water, and 0.3 ml, 0.275 ml, 0.25 ml, 0.225ml, 0.20ml, 0.175ml, 0.150ml, 0.125ml, 0.100ml, 0.075ml, 0.050 ml, 0.025ml, 0.0 ml Cys mother solution. Incubate at room temperature for 10 minutes, excite with a light source with a wavelength of 360 nm, and test its fluorescence emission spectrum. Such as image 3 As shown, the results show that the probe molecules can accurately characterize different concentrations of cysteine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com