A preparation method of minodronic acid for treating osteoporosis
A technology for minodronic acid and osteoporosis, applied in the field of medicine and chemical industry, can solve the problems of harsh conditions, slow reaction speed, and low yield, and achieve the effect of increased reaction speed, increased yield, and uniform reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
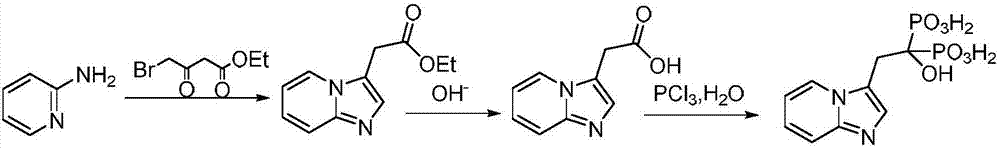
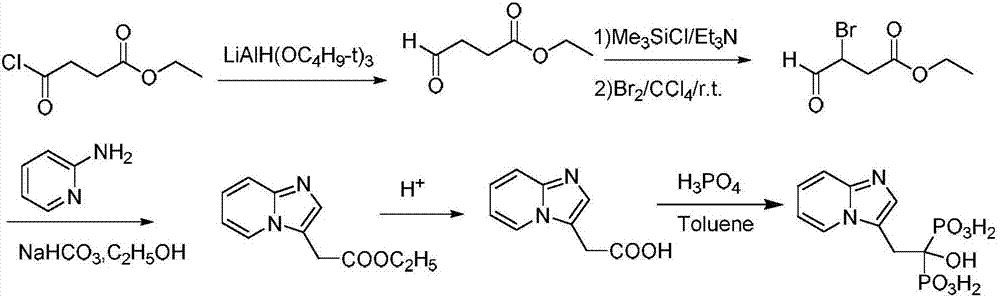
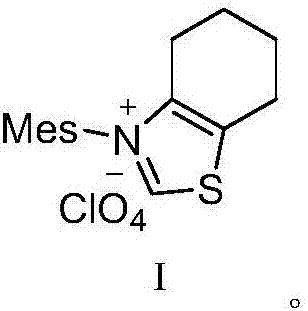
[0030] (1) 10.7g (30mmol) of the compound shown in formula I, 32.3g (250mmol) of DIEA and 20.9g (100mmol) of 4-bromo-ethyl acetoacetate were added in a 200ml flask filled with THF, stirred at room temperature for 30 minutes, Then 11.7g (125mmol) of 2-aminopyridine was added to the reaction solution, and the reaction was stirred at 45°C for 3.5 hours. TLC monitored the complete reaction of 4-bromo-ethyl acetoacetate, filtered, concentrated under reduced pressure, added water, and extracted with ethyl acetate. (50mL×3 times), washed with saturated brine (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to obtain ethyl 2-(imidazo[1,2-α]pyridin-3-yl)acetate 19.7g, yield 96.4%, purity 98.19%.
[0031]
[0032] (2) 2-(imidazo[1,2-α]pyridin-3-yl) ethyl acetate obtained in step (1) and 8.1g of lithium hydroxide in a ...
Embodiment 2
[0035] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0036] (1) 14.3g (40mmol) of compound shown in formula I, 25.8g (200mmol) of DIEA and 20.9g (100mmol) of 4-bromo-ethyl acetoacetate were added in a 200ml flask equipped with THF, stirred at room temperature for 30 minutes, Then 11.7g (125mmol) of 2-aminopyridine was added to the reaction solution, and the reaction was stirred at 40°C for 3.5 hours. TLC monitored the complete reaction of 4-bromo-ethyl acetoacetate, filtered, concentrated under reduced pressure, added water, and extracted with ethyl acetate. (50mL×3 times), washed with saturated brine (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to obtain ethyl 2-(imidazo[1,2-α]pyridin-3-yl)acetate 19.3g, yield 94.5%, purity 97.68%.
[0037](2) 2-(imidazo[1,2-α]pyridin-3-yl) ethyl acetate obtained in step (1) and 6.8g of lithium hydroxide in a mixed solvent o...
Embodiment 3
[0040] A preparation method of minodronic acid for treating osteoporosis, the method comprises the following steps:
[0041] (1) 14.3g (40mmol) of the compound shown in formula I, 38.8g (300mmol) of DIEA and 20.9g (100mmol) of 4-bromo-ethyl acetoacetate were added to a 200ml flask filled with THF, stirred at room temperature for 30 minutes, Then 11.7g (125mmol) of 2-aminopyridine was added to the reaction solution, and the reaction was stirred at 50°C for 3.5 hours. TLC monitored the complete reaction of 4-bromo-ethyl acetoacetate, filtered, concentrated under reduced pressure, added water, extracted with ethyl acetate (50mL×3 times), washed with saturated brine (50mL×3 times), combined the organic phases, removed the solvent under reduced pressure, and dried to obtain ethyl 2-(imidazo[1,2-α]pyridin-3-yl)acetate 19.1g, yield 93.7%, purity 97.9%.
[0042] (2) 2-(imidazo[1,2-α]pyridin-3-yl) ethyl acetate obtained in step (1) and 8.9g of lithium hydroxide in a mixed solvent of 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com