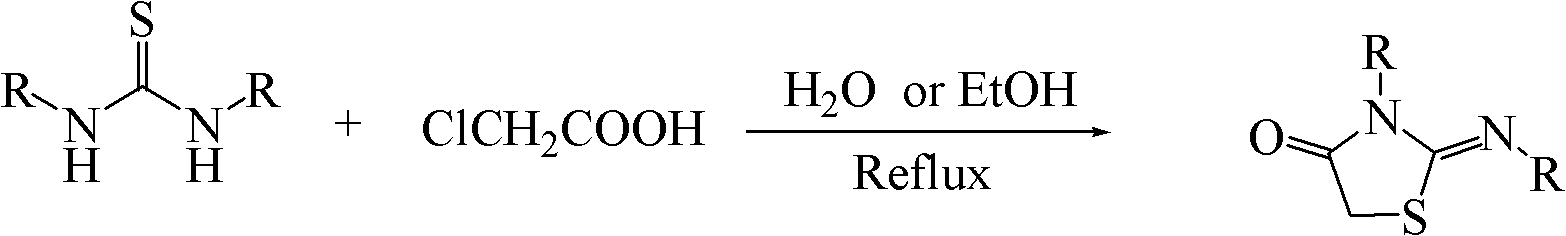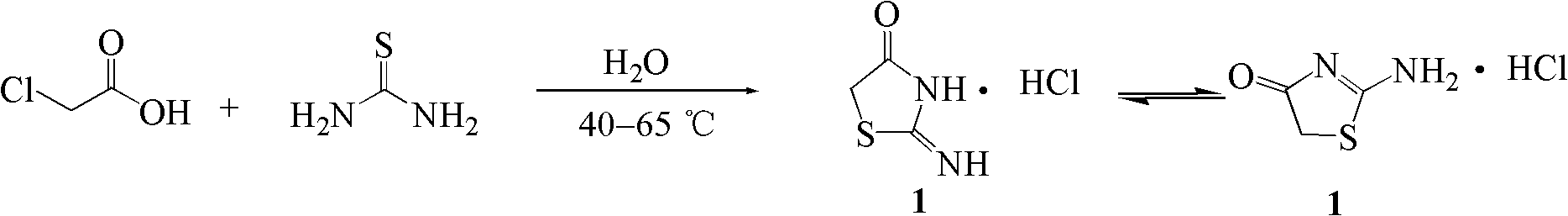Method for synthesizing 2-iminothiazolidine-4-one and derivatives thereof
An iminothiazolidine and synthesis method technology, which is applied in the field of thiazole compound synthesis, can solve problems such as inability to further modify, high toxicity of reagents, cumbersome post-processing, etc., and achieve the effect of ingenious design ideas, avoiding corrosion, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: Synthesis of 2-iminothiazolidin-4-one hydrochloride (1)
[0052]
[0053] Chloroacetic acid (9.46g, 100mmol), thiourea (7.61g, 100mmol) and water (10mL) were added successively in the reaction kettle. Stir the reaction in a water bath at 40°C, the reaction is exothermic, the reaction system becomes clear quickly, and then the temperature of the system rises, and the highest temperature reaches 72°C. After that, the temperature of the system gradually decreases, and the white solid increases. After 1 hour of reaction, the reaction temperature is constant to 40°C, and TLC is used. (Developer: chloroform-methanol 10:1) to monitor the reaction progress, iodine cylinder staining, showing that the reaction was complete, after the system was naturally cooled to room temperature, filtered with suction, the precipitate was collected and washed with a small amount of ice water, dried to obtain white crystals (9.15g) , which is 2-iminothiazolidin-4-one hydrochlori...
Embodiment 2
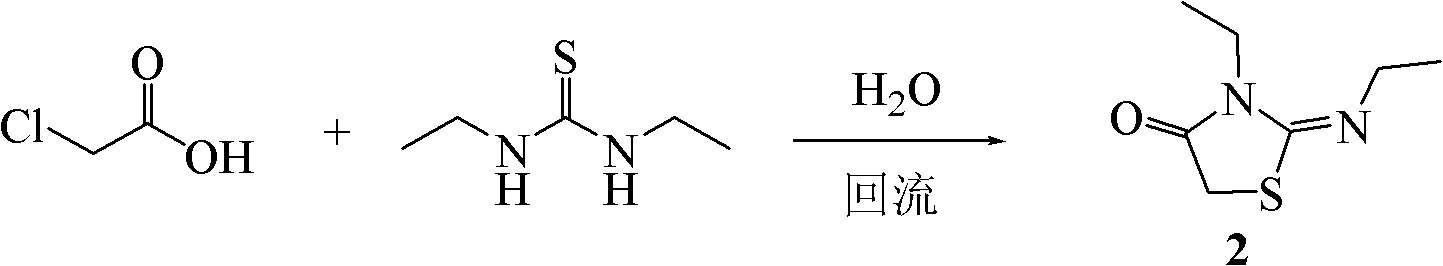
[0054] Example 2: Synthesis of 2-(ethylimino)-3-ethyl-thiazolidin-4-one (2)
[0055]
[0056] Add chloroacetic acid (2.35g, 25.0mmol), 1,3-diethylthiourea (3.05g, 23.0mmol) and water (10mL) successively in the flask, stir in an oil bath to dissolve completely, and heat to 100°C, reflux reaction for 5 hours, monitor the reaction progress with TLC (developing solvent: chloroform-methanol 20:1); after the reaction is completed, cool the reaction system to room temperature, add aqueous sodium hydroxide solution (2mol / L) to adjust the pH of the system to 12, extracted with ethyl acetate, and the obtained organic phase was washed with saturated sodium chloride and dried over anhydrous sodium sulfate. Filtrate and recover the organic solvent to obtain a yellow oily liquid, which gradually condenses into needle-like crystals (3.43g) after standing at room temperature, which is 2-(ethylimino)-3-ethyl-thiazolidin-4-one , and the yield was 86.4% (calculated as chloroacetic acid). Th...
Embodiment 3
[0057] Example 3: Synthesis of 3-n-butyl-2-(n-butylimino)thiazolidin-4-one (3)
[0058]
[0059] Chloroacetic acid (2.35g, 25.0mmol), 1,3-di-n-butylthiourea (4.7g, 25.0mmol) and water (15mL) were added successively in the flask, heated to 100°C, refluxed for 7.5 hours, TLC ( Developing agent: petroleum ether-ethyl acetate 5:1) to monitor the reaction progress, after the reaction was completed, when the system was cooled to room temperature, an oily substance was precipitated in the system, extracted with ethyl acetate, washed with saturated sodium chloride, and dried over anhydrous sodium sulfate. After filtration, the organic solvent was distilled off to obtain a yellow oily liquid (3.7 g), with a yield of 64.9% (based on chloroacetic acid). MS: 228.1(25)[M] + ; 1 H NMR (400MHz, CDCl 3 )δ: 3.92(s, 1H, HO-CH=), 3.76(s, 2H, R 1 R 2 NCH 2 CH 2 CH 2 CH 3 ), 3.69(t, 2H, R 1 R 2 NCH 2 CH2 CH 2 CH 3 ), 3.59(t, 2H, R 1 R 2 NCH 2 CH 2 CH 2 CH 3 ), 3.25(t, 2H, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com