Universal clinical test electronic data acquisition system and method
A clinical trial and electronic data technology, applied in the direction of electronic digital data processing, data processing applications, special data processing applications, etc., can solve problems such as low efficiency and poor accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
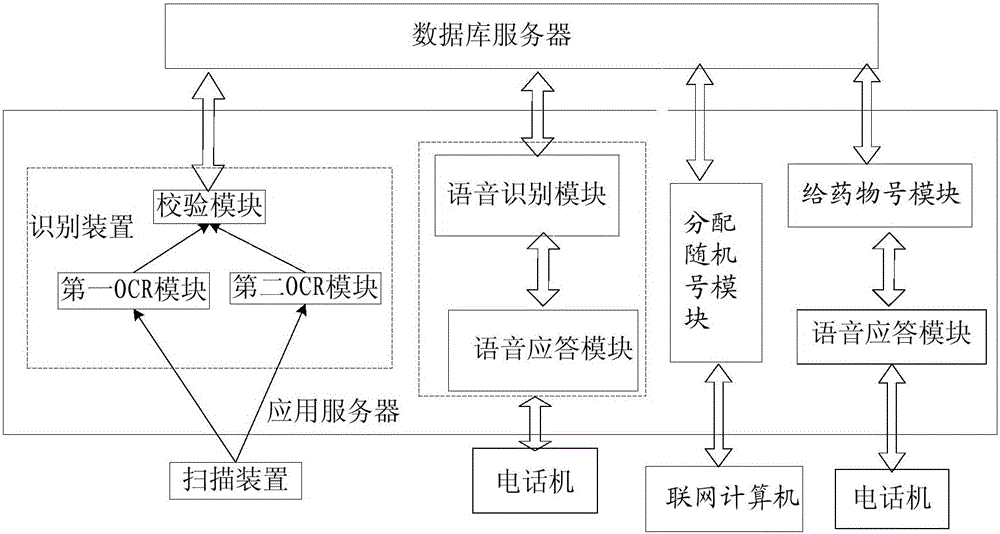
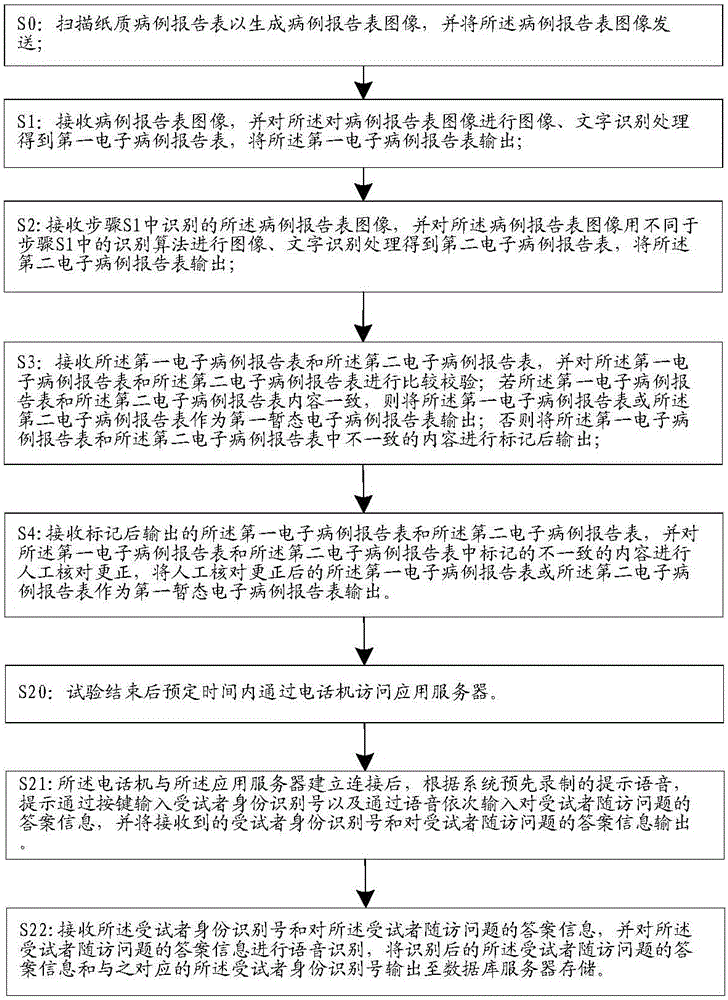
[0069] The universal clinical trial electronic data acquisition system of an embodiment of the present invention includes a client system, an application server, and a database server; wherein, the client system is used to access the application server before the trial starts, so as to realize the randomization of subjects number allocation; the client system is used to access the application server during the test to realize the drug number allocation of the subjects; the client system is also responsible for collecting test data during the test and collecting follow-up data after the test is over , and send the collected test data and collected follow-up data to the application server, and the application server receives the test data and follow-up data and sends them to the database server for storage after processing.
Embodiment 2
[0071] see figure 1 As shown, as a general-purpose test data collection system according to an embodiment of the present invention, on the basis of the above-mentioned embodiment, the client system includes a scanning device for collecting test data in the test stage, and a scanning device for collecting test data in the test stage before the test. A networked computer for assigning random numbers to subjects, a telephone for assigning drug numbers during the trial phase, and a telephone for collecting follow-up data after the trial is over, typically, a phone for assigning drug numbers during the trial phase The phone and the phone used to collect follow-up data after the trial are different phones distributed in different physical locations, where
[0072] Before the test starts, the networked computer is used to access the application server, and enter the subject information according to the prompts on the application server, and send the entered subject information to the...
Embodiment 3
[0089] As a general-purpose test data collection system in other embodiments of the present invention, the networked computer used to complete the distribution of the random number of the subjects in the above embodiment is replaced by a telephone, wherein the telephone is connected to the application server;
[0090] application server, further comprising:
[0091] database for storing system data;
[0092] The voice response module, after the telephone set is connected to the application server, sends out a prompt voice prompting the researcher to input subject information item by item according to the prompt voice recorded in advance by the system; and receives the subject input information input by the researcher. subject information, and then storing the received subject information into the database;
[0093] Assign random number module, read the subject information and according to the subject information, call the random allocation algorithm to randomly assign each su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com