Specific detection and quantification of cardiolipin and isolated mitochondria by positively charged AIE fluorogens and method of manufacturing of AIE fluorogens
A mitochondrial and cardiolipin technology, applied in fluorescence/phosphorescence, chemical instruments and methods, measurement devices, etc., can solve problems such as poor water solubility, unclear working mechanism, and difficulty in use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
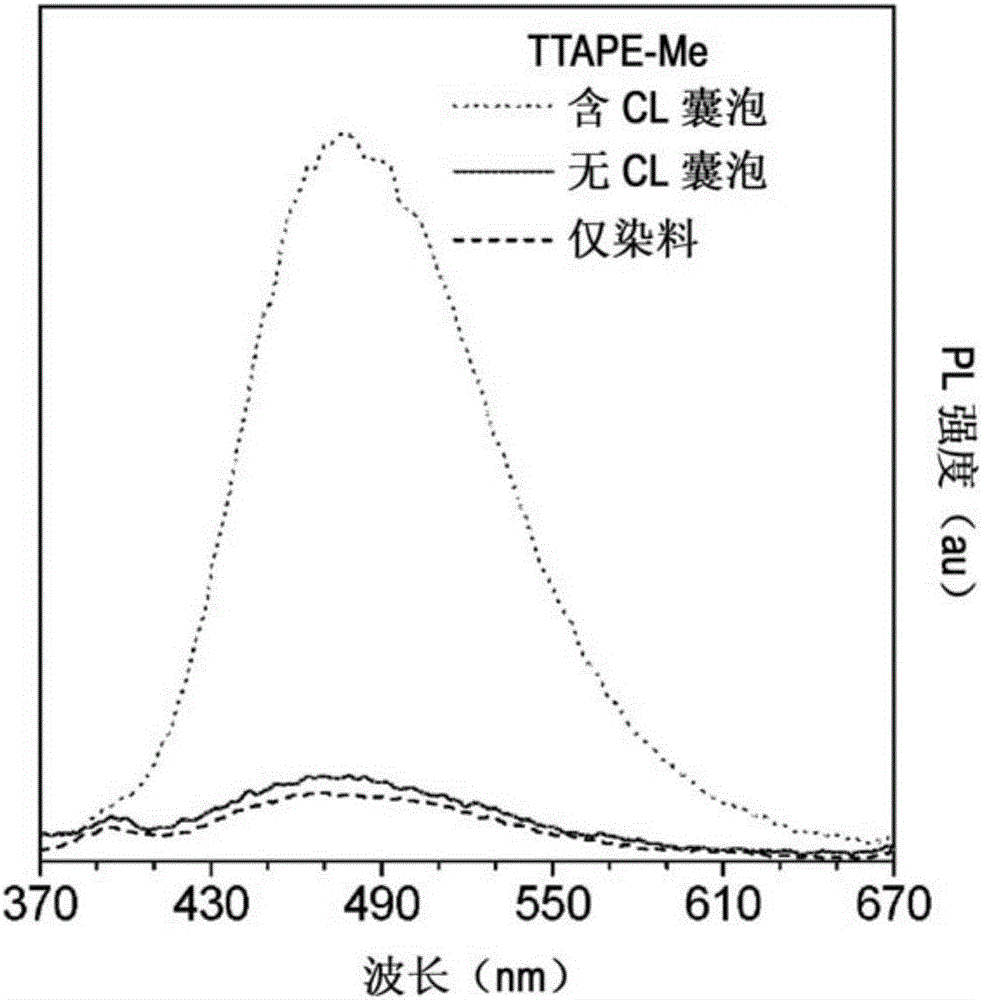
[0039] The inventors discovered that certain AIE luminophores can be used to quantify cardiolipin and isolate mitochondria. More specifically, these AIE luminophores can be applied to samples, cells, or vesicles, allowing the cells to be imaged. This allows quantification of cardiolipin or isolation of mitochondria.
[0040] The AIE luminophore may be the fluorophore TTAPE-ME. The TTAPE-ME can bind lipobinding protein, which can be a lipobinding fragment of any protein.
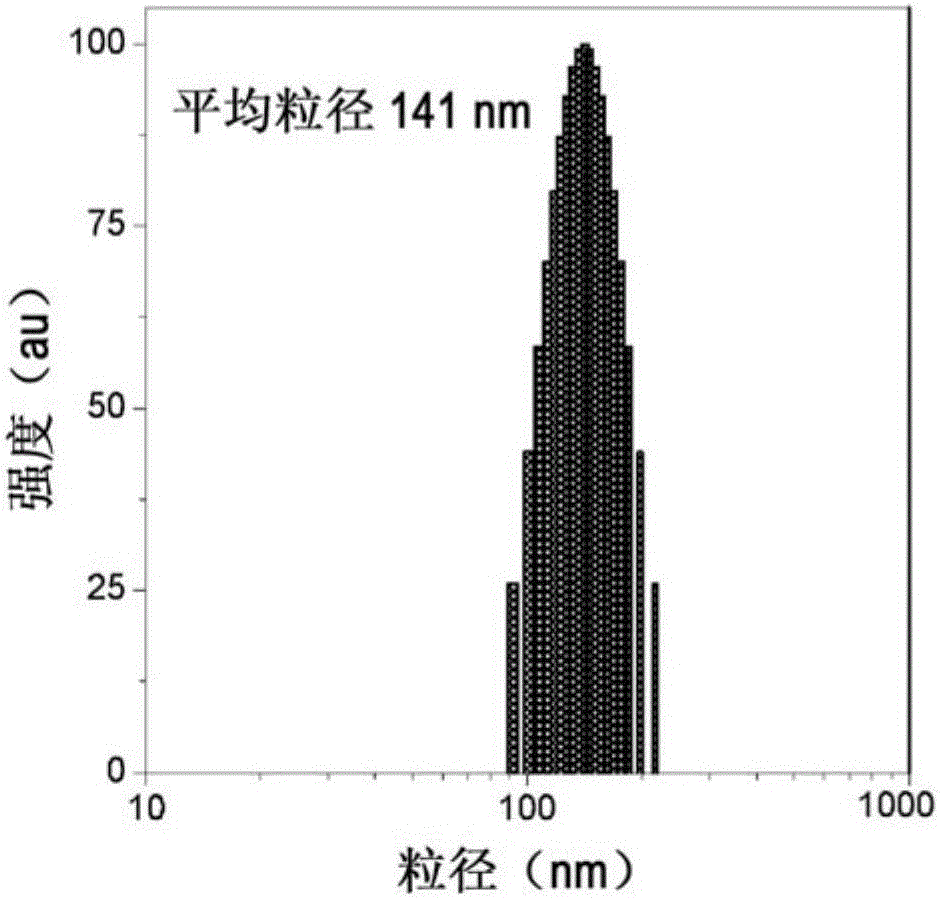
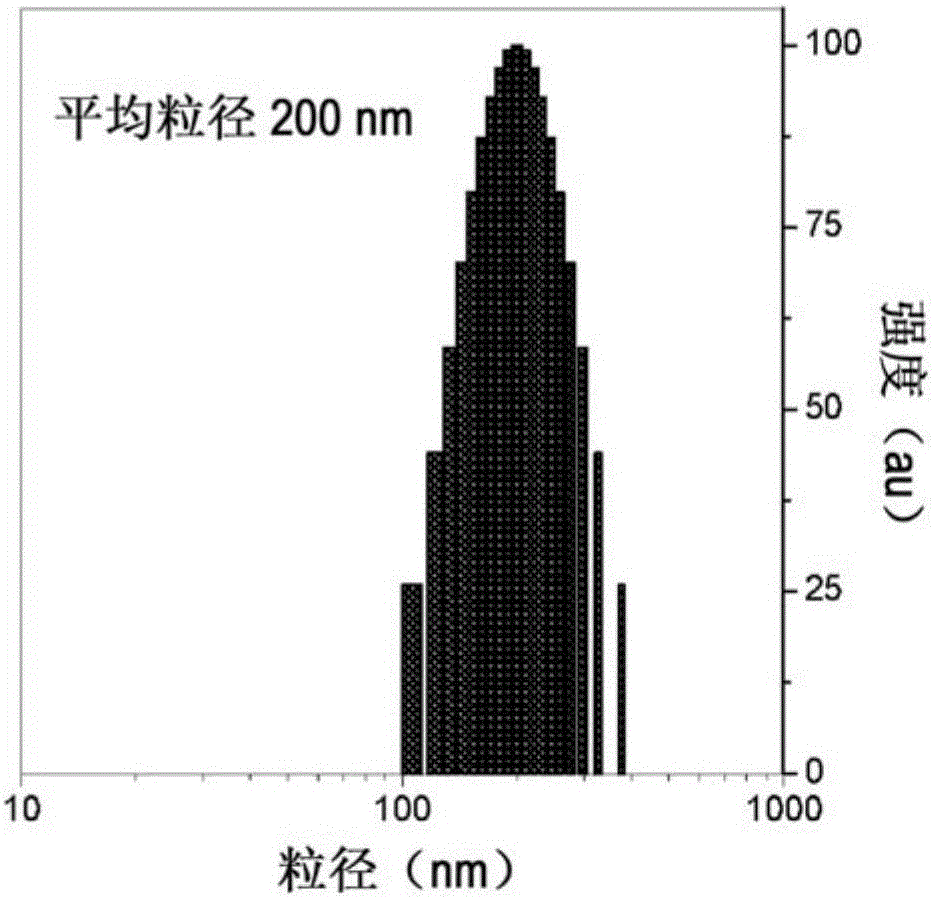
[0041] Cardiolipin can be located in the cytosol or membrane. The membrane may be in the form of a cell membrane or a lipid vesicle. The lipid vesicle may be a large unilamellar vesicle (LUV), i.e. the vesicle has a diameter of about 60nm to 800nm, 70 to 800nm, 80nm to 800nm, 90nm to 800nm, 100nm to 700nm, 200nm to 600nm, 300nm to 500nm, 400nm to 800nm, 500nm to 800nm, 600nm to 800nm, or 700nm to 800nm.
[0042] The cell membrane may be a eukaryotic cell membrane. The eukaryotic cell membrane may be a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com