Ibrutinib preparation and application thereof
A technology of ibrutinib and its use, which is applied in the field of ibrutinib for the preparation of drugs, and can solve problems such as poor prognosis
Inactive Publication Date: 2016-08-31
FOSHAN TENGRUI MEDICINE TECH CO LTD
View PDF0 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Mantle cell lymphoma is derived from CD5-positive small B cells in the immature germinal center of the lymphoid follicle mantle area. It has all the malignant characteristics of indolent lymphoma and aggressive lymphoma. Extensive extranodal infiltration, insensitive to traditional chemotherapy and radiotherapy, poor prognosis
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
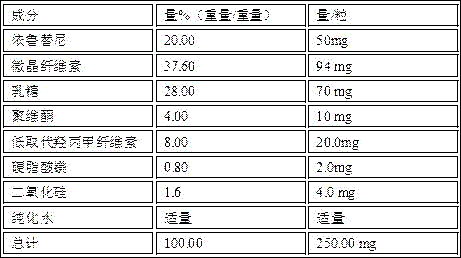
[0021] Prepare 50 mg ibrutinib capsules by the above method
[0022]
Embodiment 2
[0024] Prepare 100 mg ibrutinib capsules by the above method
[0025] Element
Embodiment 3
[0027] Prepare 140 mg ibrutinib capsules by the above method
[0028] Element
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses an ibrutinib preparation and application thereof. The ibrutinib preparation is prepared from ibrutinib, lactose, microcrystalline cellulose and a pharmaceutically-acceptable carrier, and the ibrutinib preparation with the good flowability, stability and dissolution rate can be obtained, so that the ibrutinib preparation is suitable for large industrial production. The ibrutinib preparation is used for a medicine composition for treating patients suffering from MCL and CLL, the compatibility is reasonable, medicine can be rapidly released, and the good treatment effect on diseases can be generated.
Description
technical field [0001] The invention relates to the use of ibrutinib for preparing medicine, especially for preparing tablets and capsules suitable for oral administration. [0002] Ibrutinib, molecular formula: C25H24N6O2, molecular weight: 440.5 Chemical name: 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[ 3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one. [0003] It is a three-class new drug for the treatment of patients with mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL), and is currently undergoing preclinical research. Background technique [0004] Ibrutinib is a small molecule BTK inhibitor that can covalently bind to cysteine residues in the active center of BTK, thereby inhibiting its activity. The full name of BTK is Bruton'styrosinekinase, which transmits signals in the BCR signaling pathway and cytokine receptor signaling pathway, and mediates the migration, chemotaxis and adhesion of B cells. Preclinical studies have shown that ib...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/519A61K9/48A61K9/20A61K47/38A61P35/00A61P35/02
CPCA61K31/519A61K9/2054A61K9/4866
Inventor 王雪峰韩亮
Owner FOSHAN TENGRUI MEDICINE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com