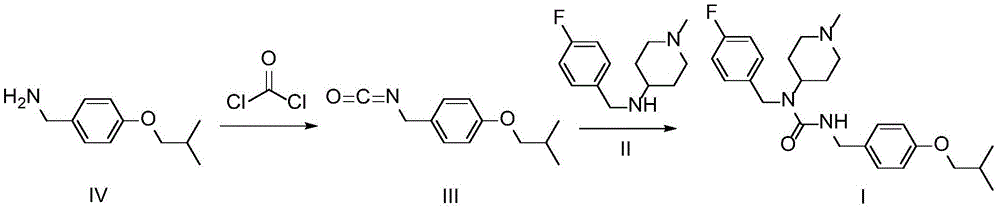
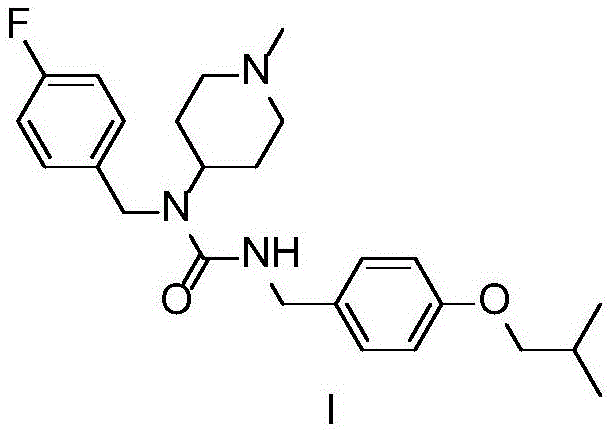
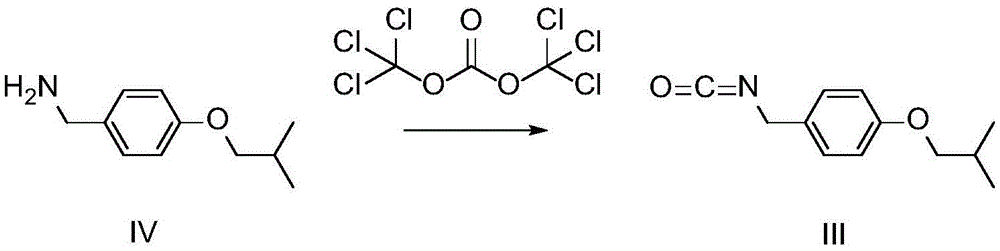
Preparation method of pimavanserin intermediate
A reaction system and a technology of isocyanatomethylbenzene are applied in the field of preparation of pimavanserin intermediates, can solve problems such as huge impact on personnel health and environment, inconvenient industrialized production, etc. Reliable quality and reasonable production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: At room temperature, add 840 mL of dichloromethane and 90 g of compound IV into a three-neck flask, stir until completely dissolved, then use an ice-salt bath to cool down to the range of -5 to 0°C and keep it stable. 160 mL of 0.625 mol / L bis(trichloromethyl)carbonate dichloromethane solution was added dropwise into the reaction solution at a rate of 20 mL / min.
[0023] Step 2: After the bis(trichloromethyl) carbonate solution is added dropwise, keep the ice-salt bath in the range of -5 to 0°C, continue to stir the reaction for 2h, then slowly warm up to room temperature within 1h, and continue at room temperature The reaction was stirred for 3h.
[0024] Step 3: add 2000mL of purified water to the reaction system and stir for 30min, remove the water phase after layering, extract twice with 500mL of dichloromethane, and combine the extracted organic phase with the organic phase of the original reaction system. The combined organic phases were washed successive...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com