Method for preparing pimavanserin crystal form C
A technology of Pimaserin and crystal form, which is applied in the field of preparation of Pimaserin Crystal Form C, can solve the problems that are not conducive to industrialization and restrict the application of Pimaserin Crystal Form C, and achieve industrialization and operation. Effects in simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The invention provides a method for preparing Pimaserin crystal form C, comprising:
[0019] The tartaric acid of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl)urea The crystalline form A of the salt was refluxed in acetone for 3-6 hours to obtain the crystalline form C of pipemaserin.
[0020] The inventive method of the present invention can realize the transformation of the Pimaserin crystal form through simple heating and reflux, without deoxidizing the solvent, without inert gas protection, and without adding seed crystals. The operation steps are simple, no harsh conditions are required, and more conducive to industrialization.
[0021] In the present invention, N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl) Form A of urea tartrate was suspended in acetone and refluxed.
[0022] The N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl)urea The ratio of the m...
Embodiment 1
[0029] 3.0g of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl)urea The crystal form A of tartrate salt was heated and refluxed in 24mL of acetone solvent for 3 hours, and the temperature was slowly lowered to 20-30°C. After vacuum filtration, the filter cake was rinsed with a certain amount of acetone, and the white filter cake was dried at 40-50°C. 2.5 g of Form C are obtained. Yield 83.3%, purity 99.84%.
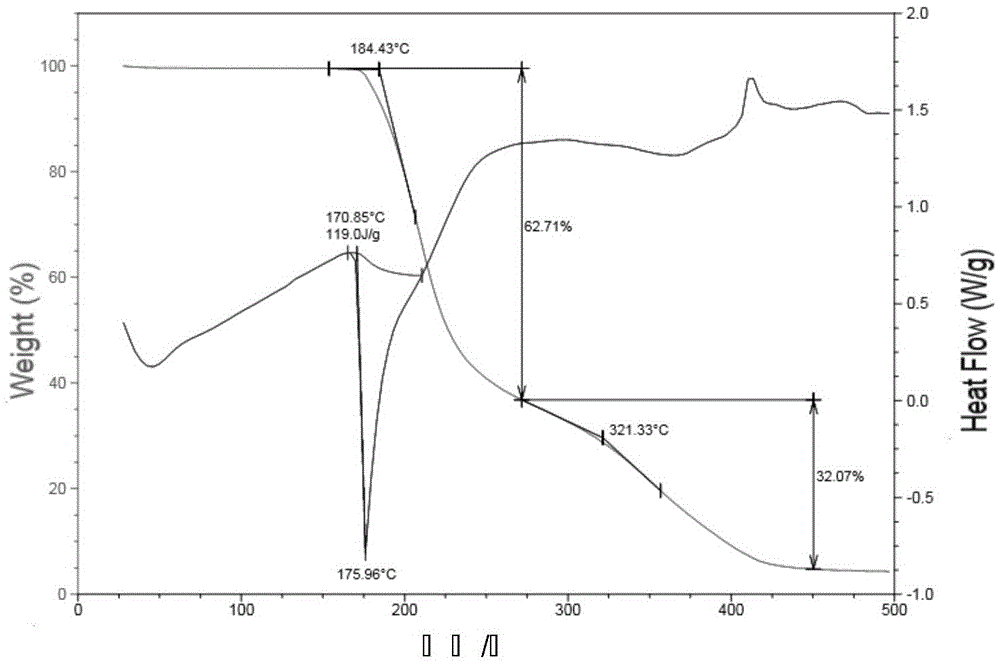
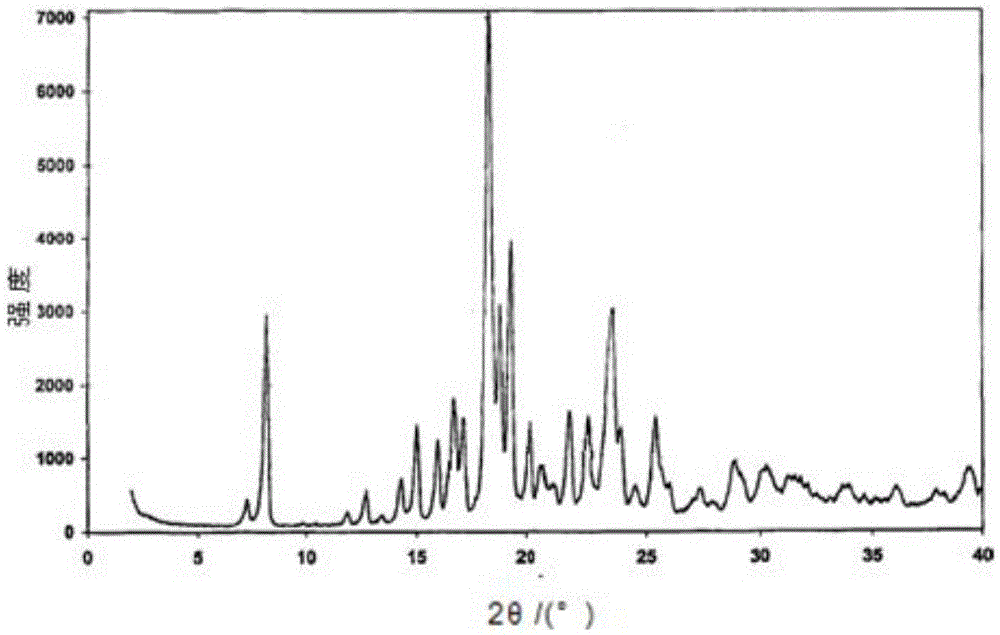
[0030] The melting point was determined to be in the range of 165-170°C, and DSC showed a melting point of 177°C. See attached for DSC-TGA diagram figure 1 . See attached for XRPD figure 2 , and the XRPD of Form C reported in the literature Figure 1 Sincerely. It can be seen that the method disclosed in the present application has prepared Pimaserin Form C.
Embodiment 2
[0032] 3.0g of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropoxy)-phenylmethyl)urea The crystal form A of tartrate salt was heated and refluxed in 30mL of acetone solvent for 6h, and the temperature was slowly lowered to 20-30°C. After vacuum filtration, the filter cake was rinsed with a certain amount of acetone, and the white filter cake was dried at 40-50°C. 2.4 g of Form C was obtained with a yield of 80.0% and a purity of 99.87%. The product was confirmed by XRPD detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com