Atosiban acetate impurities and preparation and detection methods
A technology of atosiban acetate and impurities, which is applied in the field of active polypeptide impurities and its preparation, and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
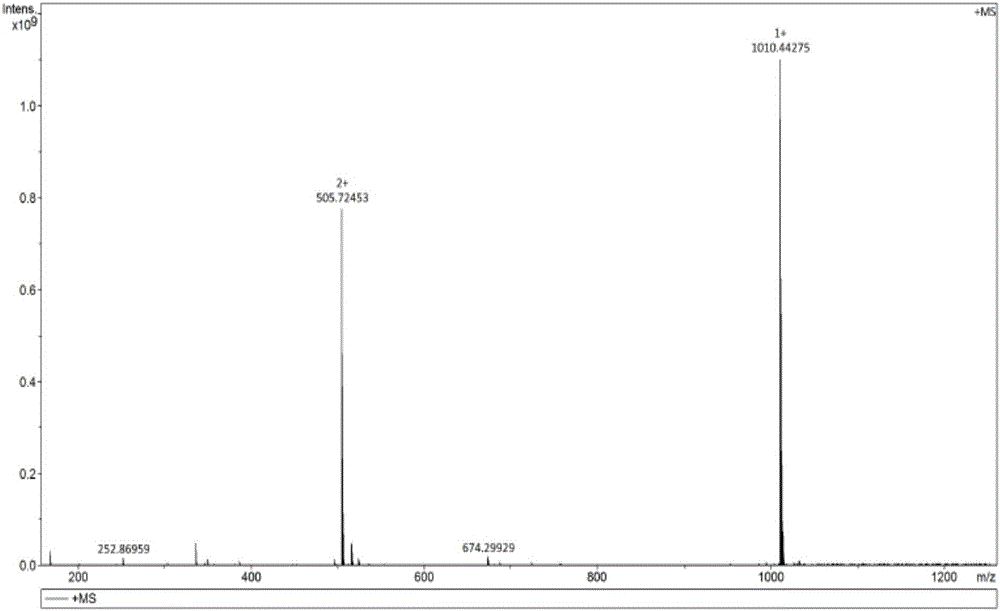
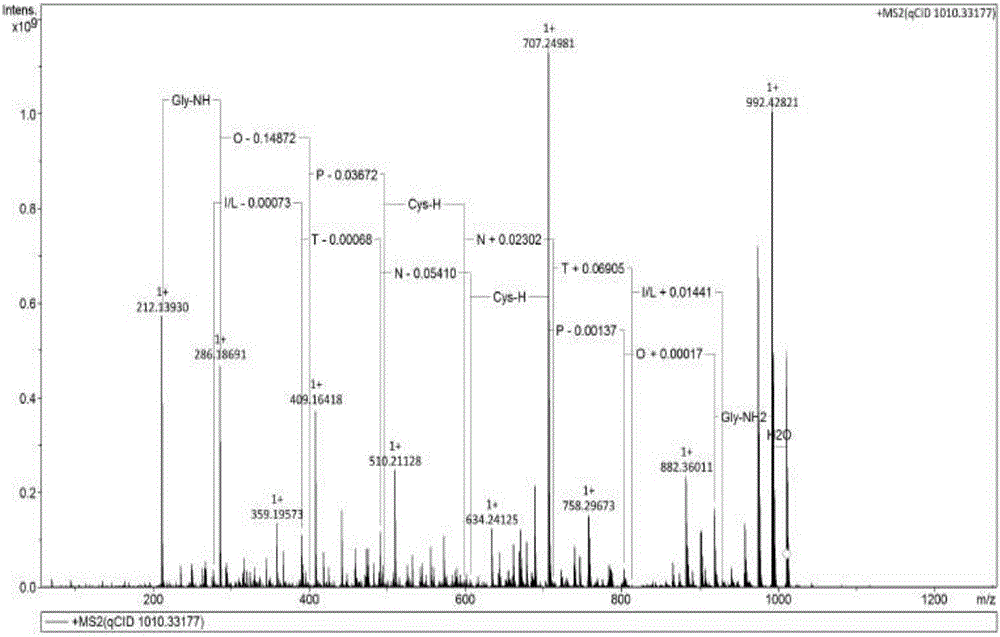
[0101] [embodiment 1] the preparation of impurity A
[0102](1) Weigh 2 g of atosiban acetate finished product, dissolve it ultrasonically with 80 ml of high-purity water, add 20 ml of 30% hydrogen peroxide under stirring, and perform an oxidation reaction for 2 hours to obtain a solution of impurity A;
[0103] (2) The oxidized solution is filtered through a 0.45um filter membrane, and purified by a semi-preparative high-performance liquid chromatograph, and the used chromatographic column is C 18 Filler, (10um, 21mm×250mm); each loading volume is 10ml (0.2g), with acetonitrile as mobile phase A, 0.05% TFA / H 2 O is the mobile phase B, the detection wavelength is 220nm, keep 15% mobile phase A for 10min, and change the mobile phase A to 45% within 10min to 50min, collect the component for 36-39min, which is impurity A component, and concentrate at 40°C Acetonitrile was removed, then bottled and freeze-dried to obtain impurity A. The purity of impurity A determined by liquid ...
Embodiment 2
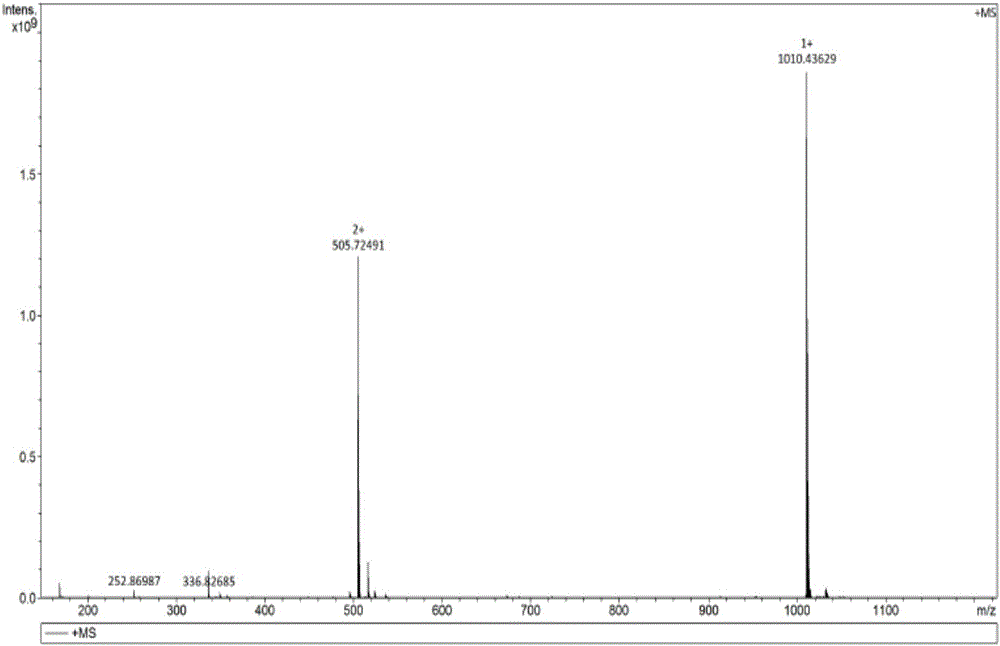
[0104] [embodiment 2] the preparation of impurity A
[0105] (1) Weigh 2 g of atosiban acetate finished product, dissolve it ultrasonically with 80 ml of high-purity water, add 10 ml of 30% hydrogen peroxide under stirring, and perform an oxidation reaction for 1 hour to obtain a solution of impurity A;
[0106] (2) The oxidized solution is filtered through a 0.45um filter membrane, and purified by a semi-preparative high-performance liquid chromatograph, and the used chromatographic column is C 18 Filler, (10um, 21mm×250mm); each loading volume is 10ml (0.2g), with acetonitrile as mobile phase A, 0.05% TFA / H 2 O is the mobile phase B, the detection wavelength is 220nm, keep 15% mobile phase A for 10min, and change the mobile phase A to 45% within 10min to 50min, collect the component for 36-39min, which is impurity A component, and concentrate at 35°C Acetonitrile was removed, then bottled and freeze-dried to obtain impurity A. The purity of impurity A is 99.8% as determine...
Embodiment 3
[0107] [embodiment 3] the preparation of impurity A
[0108] (1) Weigh 2 g of atosiban acetate finished product, dissolve it ultrasonically with 80 ml of high-purity water, add 30 ml of 30% hydrogen peroxide under stirring, and perform an oxidation reaction for 3 hours to obtain a solution of impurity A;
[0109] (2) The oxidized solution is filtered through a 0.45um filter membrane, and purified by a semi-preparative high-performance liquid chromatograph, and the used chromatographic column is C 18 Filler, (10um, 21mm×250mm); each loading volume is 10ml (0.2g), with acetonitrile as mobile phase A, 0.05% TFA / H 2 O is the mobile phase B, the detection wavelength is 220nm, keep 15% mobile phase A for 10min, and change the mobile phase A to 45% within 10min to 50min, collect the component for 36-39min, which is impurity A component, and concentrate at 45°C Acetonitrile was removed, then bottled and freeze-dried to obtain impurity A. The purity of impurity A is 99.8% as determin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com