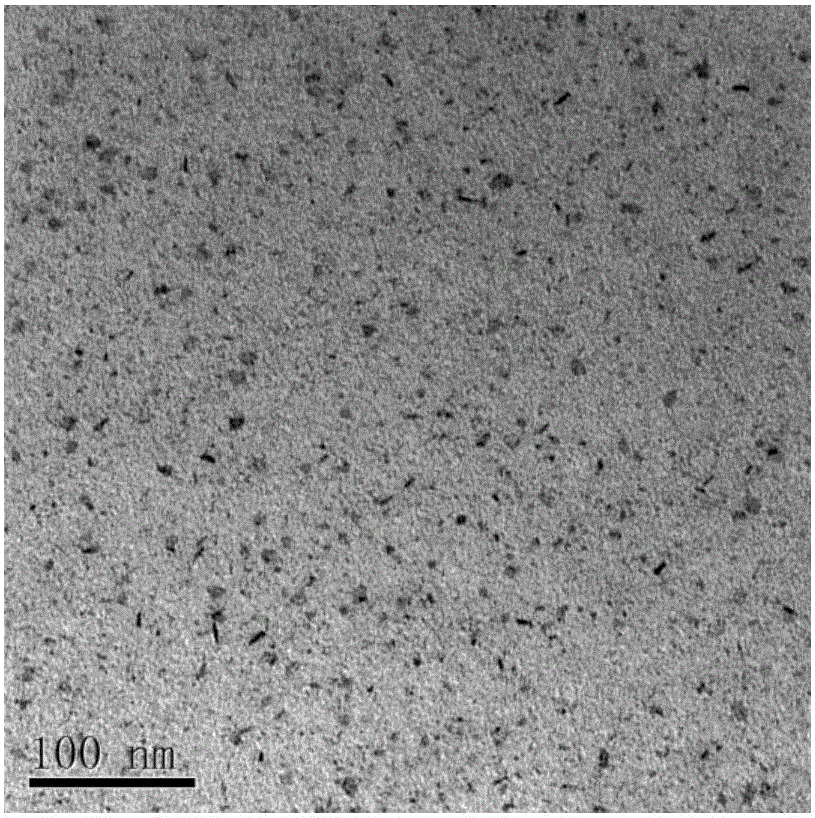
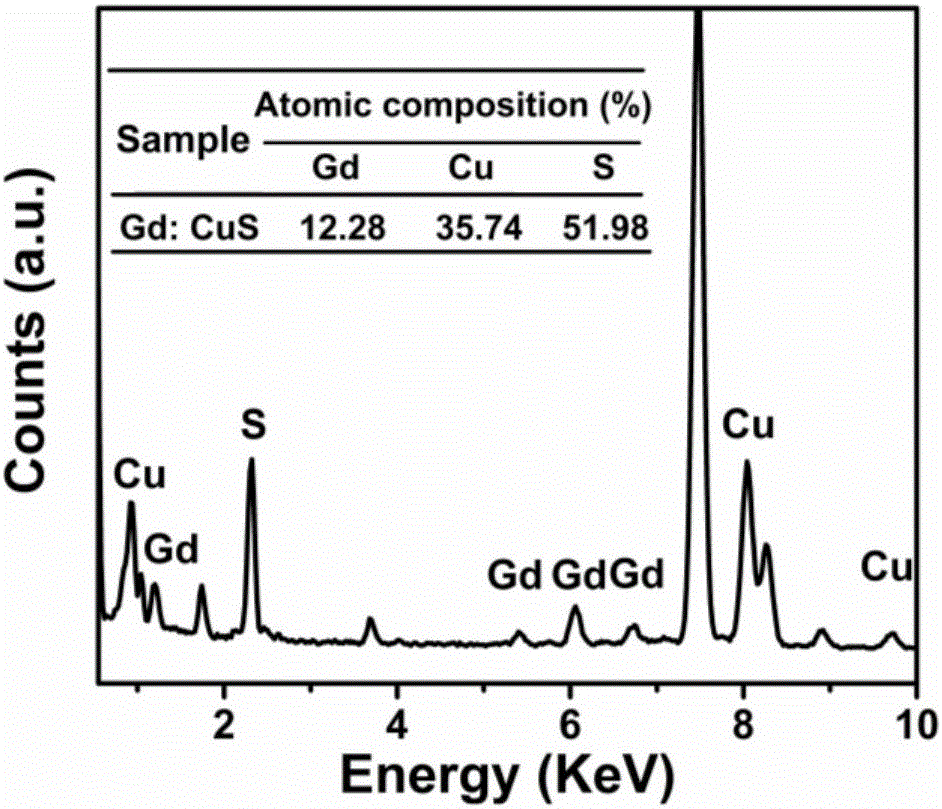
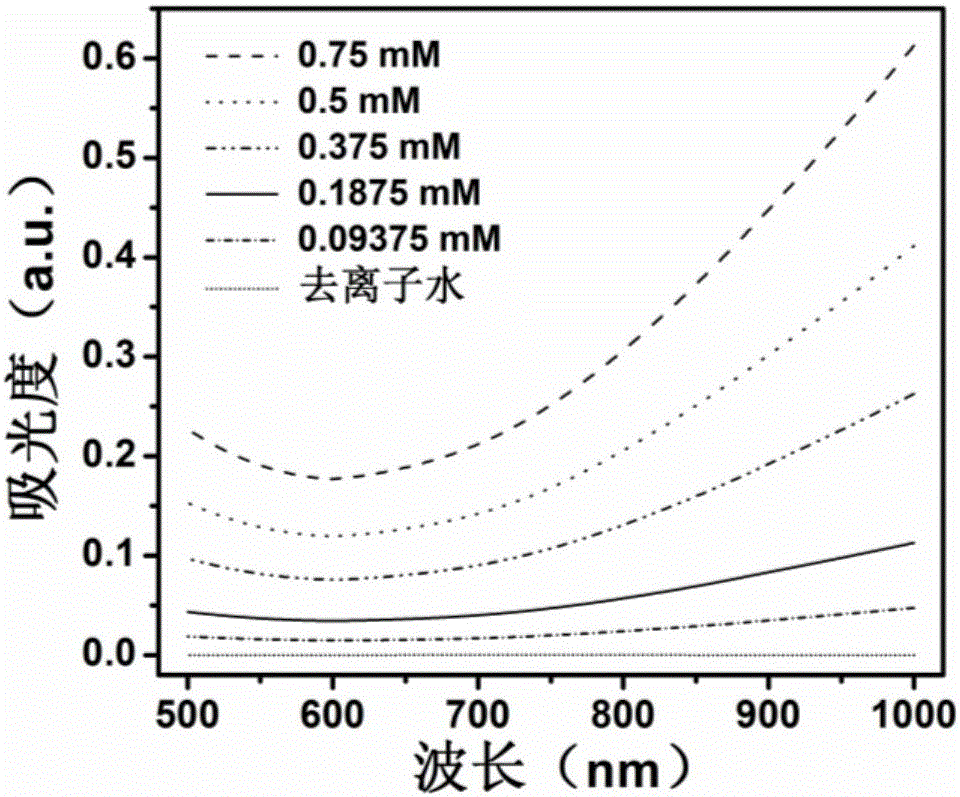
Protein biological template-based gadolinium-doped copper sulfide nano-particles and preparation method thereof
A technology of nanoparticles and biological templates, which can be applied in preparations for in vivo experiments, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of the biological safety of the surface ligands of copper sulfide nanoparticles to be considered, etc. Achieve the effects of convenient mass production, high relaxation efficiency, and easy concentration and collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Accurately weigh 0.05mmol bovine serum albumin powder with an electronic balance and pour it into a 50mL single-mouth bottle, add 10mL phosphate buffer (0.01M), dissolve by ultrasonic vibration, and obtain a clear and transparent bovine serum albumin solution.
[0033] (2) Take 3mmol of CuCl respectively 2 2H 2 O and 5 mmol GdCl 3 ·6H 2 O was added to the solution in (1), and the stirring was continued for 5 min at room temperature.
[0034] (3) Prepare a sodium hydroxide solution with a concentration of 1M, add it to the reaction flask, and adjust the pH of the system to 8.
[0035] (4) Configure Na with a concentration of 4mmol / mL 2 S·9H 2 O solution, 0.4 mL was taken out and added to the above reaction system, and the reaction was continued for 12 h at 25 °C.
[0036] (5) After the reaction, the solution was taken out, added to a dialysis bag with a molecular weight cut-off of 8000-14000, placed in a beaker filled with secondary water, and dialyzed for 24 h...
Embodiment 2
[0039] (1) Accurately weigh 0.1 mmol of bovine serum albumin powder with an electronic balance and pour it into a 50 mL single-mouth bottle, add 10 mL of phosphate buffer (0.01 M), and dissolve by ultrasonic vibration to obtain a clear and transparent bovine serum albumin solution.
[0040] (2) Take 4.5mmol of CuCl respectively 2 2H 2O and 5 mmol GdCl 3 ·6H 2 O was added to the solution in (1), and the stirring was continued for 5 min at room temperature.
[0041] (3) Prepare a sodium hydroxide solution with a concentration of 1M, add it to the reaction flask, and adjust the pH of the system to 12.
[0042] (4) Configure Na with a concentration of 4mmol / mL 2 S·9H 2 O solution, 0.4 mL was taken out and added to the above reaction system, and the reaction was continued at 37° C. for 4 h.
[0043] (5) After the reaction, the solution was taken out, added to a dialysis bag with a molecular weight cut-off of 8000-14000, placed in a beaker filled with secondary water, and dial...
Embodiment 3
[0046] (1) Accurately weigh 0.15mmol bovine serum albumin powder with an electronic balance and pour it into a 50mL single-mouth bottle, add 10mL phosphate buffer (0.01M), dissolve by ultrasonic vibration, and obtain a clear and transparent bovine serum albumin solution.
[0047] (2) Take 6mmol of CuCl respectively 2 2H 2 O and 5 mmol GdCl 3 ·6H 2 O was added to the solution in (1), and the stirring was continued for 5 min at room temperature.
[0048] (3) Prepare a sodium hydroxide solution with a concentration of 1M, add it to the reaction flask, and adjust the pH of the system to 10.
[0049] (4) Configure Na with a concentration of 4mmol / mL 2 S·9H 2 O solution, 0.4 mL was taken out and added to the above reaction system, and the reaction was continued at 50° C. for 12 h.
[0050] (5) After the reaction, the solution was taken out, added to a dialysis bag with a molecular weight cut-off of 8000-14000, placed in a beaker filled with secondary water, and dialyzed for 48...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com