Application of bacterial filamentous hemagglutinin Fha recombinant protein
A technology of hemagglutinin and recombinant protein, which is applied in the application field of bacterial filamentous hemagglutinin Fha recombinant protein, and can solve the problems of no effective immune prevention and control methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
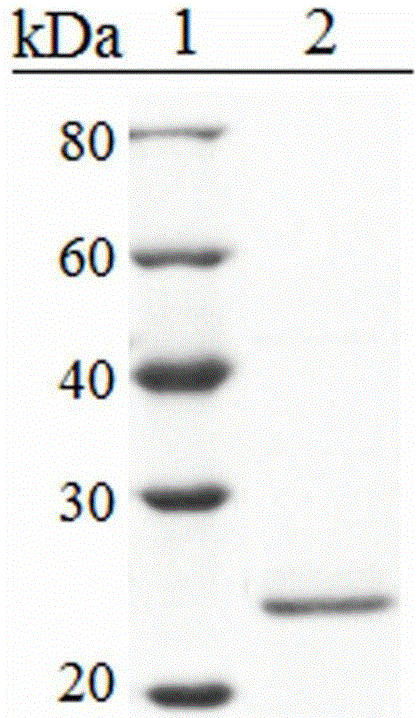
[0017] Preparation of Fha Recombinant Protein rFha
[0018] Step 1) Construction of the expression vector pEtFha of rFha:
[0019] The sequence of Pseudomonas fluorescens Fha has been reported (GenBank accession number. WP_014719704.1). The sequence contains a haemagglutination activity domain consisting of amino acids 1-684.
[0020] The construction of pEtFha is as follows: Pseudomonas fluorescens TSS1 is used as a template, and the hemagglutination activity domain of Fha is amplified by primers F1 / R1. The PCR conditions were: 94°C for 60s to pre-denature template DNA, then 94°C for 40s, 62°C for 60s, 72°C for 60s, 30 cycles. PCR products were purified with corresponding kits from Tiangen. The expression vector pET259 (see HuYH, Zheng WW, Sun L. Identification and molecular analysis of a ferritin subunit from red drum (Sciaenops ocellatus) for the construction process of pET259. Fish Shellfish Immunol 2010; 28:678-86) with restriction enzyme SwaI After digestion, connect...
Embodiment 2
[0028] Immunization application of rFha as a vaccine
[0029] Step 1) Preparation of adjuvant and vaccine mixture.
[0030] Preparation of adjuvant control solution: mix 5% (mass ratio) NaOH and 5% (mass ratio) Al 2 (SO 4 ) 3 Mix in a 2:5 volume ratio, and centrifuge the mixture at 10,000g for 5 minutes. Suspend the precipitate in PBS to 0.2mg / ml, which is the adjuvant. Mix equal volumes of PBS and adjuvant, which is the adjuvant control solution.
[0031] Vaccine mixture preparation: the rFha purified in the above-mentioned Example 1 was diluted to 200ug / ml in PBS, and the diluted protein was mixed with the adjuvant control solution in equal volumes to obtain the rFha vaccine mixture.
[0032] The composition of the PBS is by weight percentage: 0.8% NaCl, 0.02% KCl, 0.358% Na 2 HPO 4 .12H 2 O, 0.024% NaH 2 PO 4 , and the balance is water.
[0033] Step 2) Immunization application of the vaccine. 60 turbots (each weighing about 14 g) were randomly divided into 2 gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com