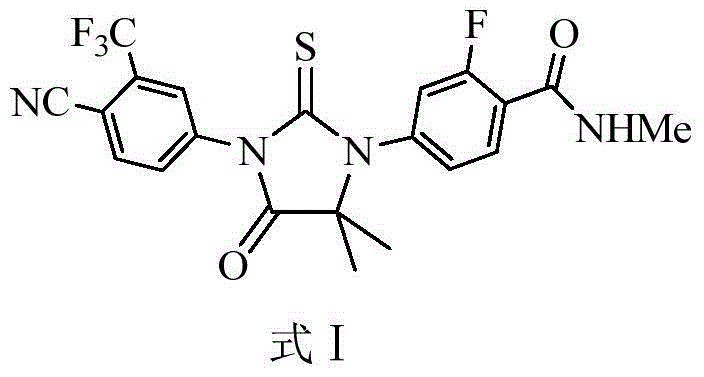
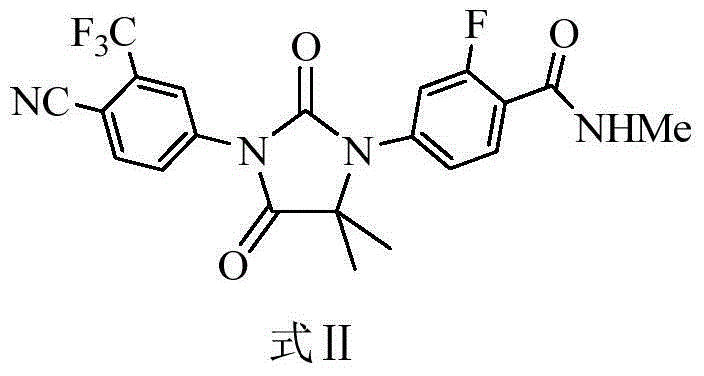
Enzalutamide purification method
A kind of technology of enzalutamide and purification method, applied in the direction of organic chemistry, etc., can solve the problems of difficult purification, difficult to remove, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Enzalutamide is purified by a mixed solvent of n-butanol and cyclohexane
[0028] Add 200 g of enzalutamide crude product (HPLC purity 99.38%) and 3,000 ml of n-butanol into a three-necked flask, stir, under nitrogen protection, heat to reflux, add 2,000 ml of cyclohexane after dissolving, slowly drop to room temperature, stir and crystallize for 3 hours, After filtration, the filter cake was vacuum-dried at 55±3° C. for 6 hours to obtain a purified product of enzalutamide, 153.5 g of a white solid, with a purity of 99.76% (HPLC area normalization method).
Embodiment 2
[0029] Embodiment 2 Enzalutamide is purified through isopropanol
[0030] Add 80 g of crude enzalutamide (HPLC purity 99.37%) and 1600 ml of isopropanol into a three-necked flask, stir, under nitrogen protection, heat to reflux, stop heating after dissolving, slowly cool down to room temperature, stir and crystallize for 3 hours. After filtration, the filter cake was vacuum-dried at 55±3° C. for 12 hours to obtain 72.0 g of a white solid with a purity of 99.41% (HPLC area normalization method).
Embodiment 3-15
[0031] Embodiment 3-15 Enzalutamide is purified by other solvents
[0032] Referring to the method of Example 2, isopropanol was replaced by other solvents for purification to obtain Examples 3-15, and the results of Examples 1-15 are summarized in Table 1.
[0033] Table 1 Enzalutamide purification results
[0034]
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com