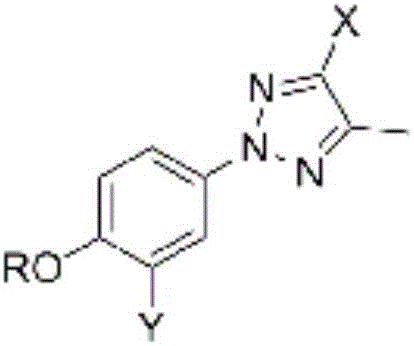
Xanthine oxidase inhibitor
A technology of compounds and solvates, applied in the field of xanthine oxidase inhibitors, can solve problems such as no reports yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
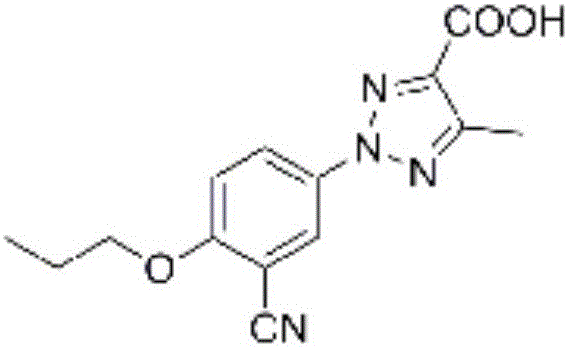
[0066] Example 1: Preparation of 2-(3-cyano-4-n-propoxyphenyl)-5-methyl-2H-1,2,3,-triazole-4-carboxylic acid (compound 1)
[0067] (1) Diazotization and condensation:
[0068] Crude 5-amino-2-n-propoxybenzonitrile (0.48 g, 2.6 mmol), 15% HCl solution (5 mL), was added to H 2 O (5mL), stirred for 30 minutes under ice-bath conditions; NaNO was slowly added dropwise to the system 2 (0.27g, 3.0mmol) of the aqueous solution, dripping finished, continue to react in ice bath for 30 minutes; Add the ethanol solution of ethyl acetoacetate (0.39g, 3mmol) in the system, then use the saturated aqueous solution of sodium acetate to adjust the pH of the system to Neutral, reacted for 30 minutes; a yellow solid was precipitated, suction filtered, the filter cake was washed with water, and dried to obtain crude ethyl 2-(3-cyano-4-n-propoxyphenylhydrazone)-3-oxobutanoate, which was collected as The rate is 83%.
[0069] (2) ring closure:
[0070] Crude ethyl 2-(3-cyano-4-n-propoxyphenylhyd...
Embodiment 2
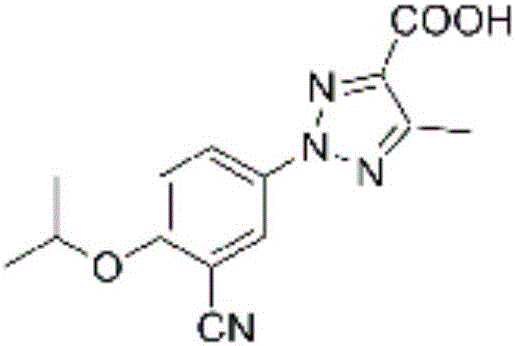
[0074] Example 2: Preparation of 2-(3-cyano-4-isopropoxyphenyl)-5-methyl-2H-1,2,3,-triazole-4-carboxylic acid (compound 2)
[0075] Except using different raw materials, 2-(3-cyano-4-isopropoxyphenyl)-5-methyl-2H-1,2,3,-triazole was prepared in the same manner as in Example 1 -4-formic acid (compound 2), the total yield is 68% (based on raw material 4-isopropoxy-3-cyanoaniline), its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 3
[0076] Example 3: Preparation of 2-(3-cyano-4-n-butoxyphenyl)-5-methyl-2H-1,2,3,-triazole-4-carboxylic acid (compound 3)
[0077] Except using different raw materials, 2-(3-cyano-4-n-propoxyphenyl)-5-methyl-2H-1,2,3,-triazole was prepared in the same manner as in Example 1 -4-formic acid (compound 3), the total yield is 69% (based on raw material 4-n-butoxy-3-cyanoaniline), its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com