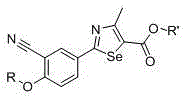
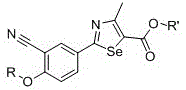
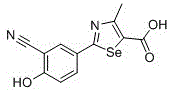
2-(3-cyano-4-substituted phenyl)-4-methyl-1,3-selenazole-5-carboxylic acid and its ester compounds and preparation method
A kind of compound, the technology of allyloxy phenyl, applied in the field of medicine, can solve the problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Preparation of 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid (compound 1)
[0108] (1) Addition: 0.79g selenium powder (10mmol) was mixed with 0.46g sodium borohydride (12mmol), the system was vacuumed and then filled with nitrogen for protection; under ice bath, 20mL absolute ethanol was added to prepare sodium selenium hydride solution; Add 1.2g (10mmol) of p-hydroxybenzonitrile, reflux reaction for 6h; dropwise add 6M HCl solution to make the system acidic, continue the reaction for 1h; add 100mLH 2 O, stirred in an ice bath for 0.5h, filtered, and dried to obtain the crude product of 4-hydroxyselenobenzamide with a yield of 60%. The crude product was directly used in the next reaction without purification.
[0109] (2) Cycling: 2.0g (10mmol) of 4-hydroxyselenobenzamide crude product in 5mL ethanol solution, stirring and heating to reflux, adding 1.65g (10mmol) of ethyl 2-chloroacetoacetate dropwise, and continuing to reflux for 4h...
Embodiment 2
[0114] Example 2: Preparation of 2-(3-cyano-4-ethoxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid (compound 2)
[0115] 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid ethyl ester was prepared in the same manner as in Example 1.
[0116] Alkylation: crude 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-selenazole-5-carboxylate ethyl ester 336mg (1mmol), potassium carbonate 138mg (1mmol), 5mg Potassium iodide was mixed with 2mL DMF, and 108 mg (1 mmol) of bromoethane was added dropwise under stirring at room temperature: the reaction solution was heated to 35°C, and reacted for 8 hours; after cooling, it was poured into five times the amount of water, and the solid was precipitated, filtered with suction, washed with water, and dried to obtain 2 -Crude ethyl (3-cyano-4-ethoxyphenyl)-4-methyl-1,3-selenazole-5-carboxylate, yield 83%. The crude product was directly used in the next reaction without purification.
[0117] Hydrolysis: 2-(3-cyano-4-ethoxyphenyl)-...
Embodiment 3
[0119] Example 3: Preparation of 2-(3-cyano-4-propoxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid (compound 3)
[0120] In addition to using the corresponding alkylating agent in the alkylation step, the same method as in Example 2 was used to prepare 2-(3-cyano-4-propoxyphenyl)-4-methyl-1,3- Selenazole-5-carboxylic acid (compound 3), the total yield is 17% (based on raw material p-hydroxybenzonitrile), its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com