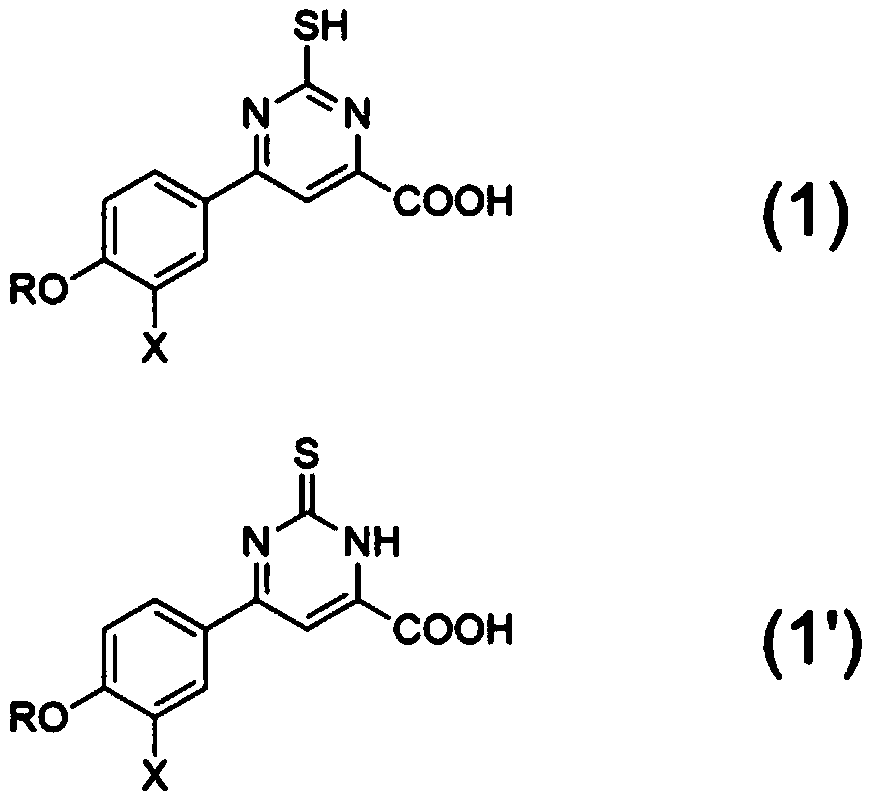
6-(3,4-substituted phenyl)-2-pyrimidinethiol-4-formate compound as well as preparation method and application thereof
A technology for mercaptopyrimidine and compound, which is applied to 6--2-mercaptopyrimidine-4-carboxylic acid compound and its preparation, and the field of medicine for treating gout, achieves the effects of good yield and simple and feasible preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
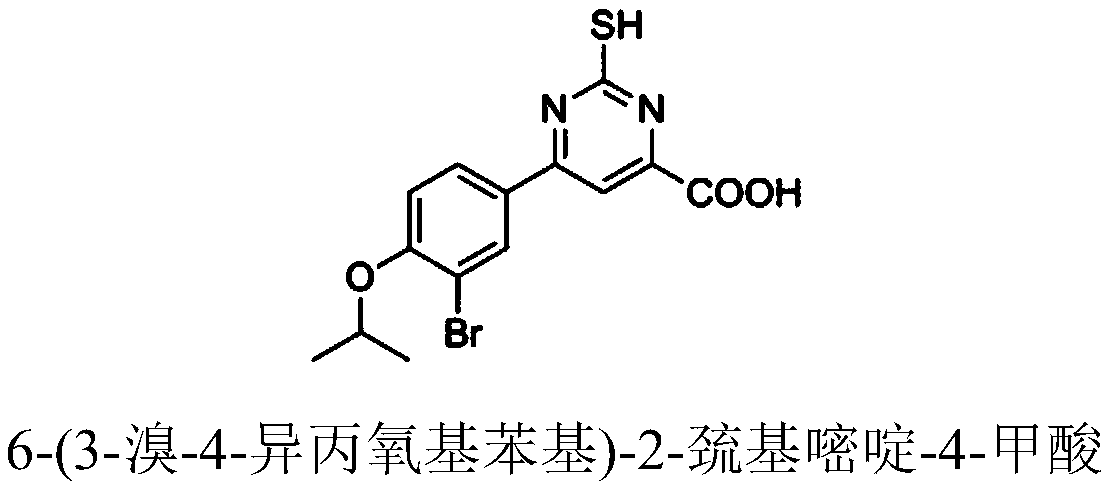
[0072] Example 1: Preparation of 6-(3-bromo-4-isopropoxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid (Compound 1)
[0073] (Z)-4-(3-bromo-4-isopropoxyphenyl)-2-hydroxy-4-oxobut-2-enoic acid (0.66g, 2mmol), thiourea (0.76g, 10mmol) ), added to glacial acetic acid (30 mL), heated to 100 °C and reacted for 8 hours. After the system was cooled to room temperature, the organic solvent was removed under reduced pressure, and the solid was purified by column chromatography to obtain 6-(3-bromo-4-isopropoxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid.
[0074] The yield of 6-(3-bromo-4-isopropoxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid (compound 1) is 66%, and its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 2
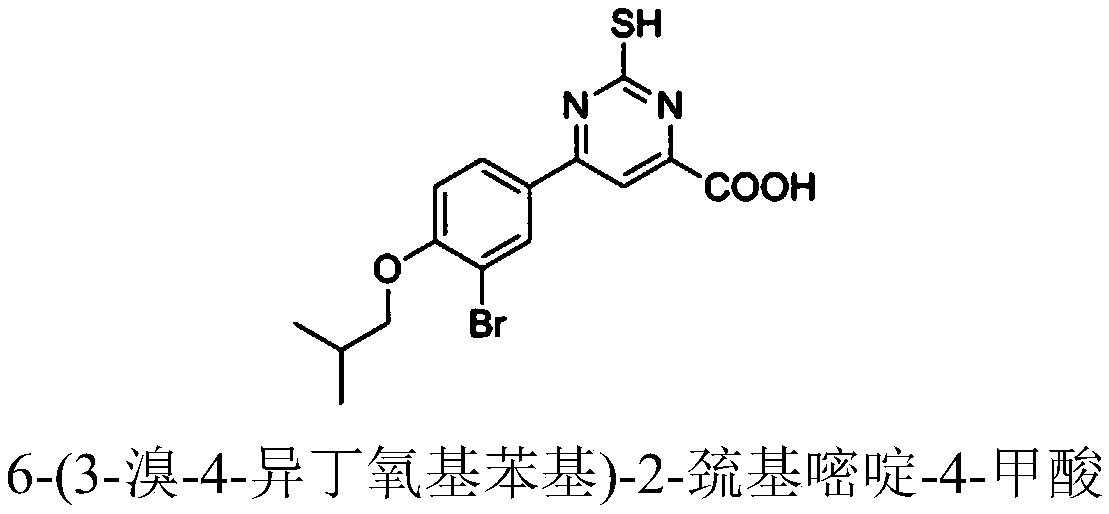
[0075] Example 2: Preparation of 6-(3-bromo-4-isobutoxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid (compound 2)
[0076] 6-(3-Bromo-4-isobutoxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid ( Compound 2), the yield is 71%, its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
Embodiment 3
[0077] Example 3: Preparation of 6-(3-bromo-4-isoamyloxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid (compound 3)
[0078] 6-(3-Bromo-4-isoamyloxyphenyl)-2-mercaptopyrimidine-4-carboxylic acid (Compound 3) was prepared in the same manner as in Example 1, the yield was 67%, and its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com