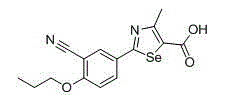
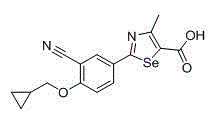
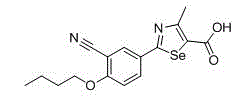
2-(3-cyano-4-substituted phenyl)-4-methyl-1, 3-selenazole-5-formic acid and formate compounds and preparation method thereof
A compound, the technology of ethyl formate, which is applied in the field of medicine and can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: Preparation of 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid (Compound 1)
[0108] (1) Addition: 0.79 g of selenium powder (10 mmol) was mixed with 0.46 g of sodium borohydride (12 mmol), and the system was evacuated and then filled with nitrogen for protection; under ice bath, 20 mL of absolute ethanol was added to obtain selenium hydrochloride Sodium solution; add 1.2 g (10 mmol) of p-hydroxybenzonitrile, reflux for 6 h; add 6 M HCl solution dropwise to make the system acidic, and continue the reaction for 1 h; add 100 mL H 2 O, stirred in an ice bath for 0.5 h, filtered, and dried to obtain the crude product of 4-hydroxyselenobenzamide with a yield of 60%. The crude product was directly used in the next reaction without purification.
[0109] (2) Cyclization: 2.0 g (10 mmol) of crude 4-hydroxyselenobenzamide in 5 mL of ethanol, stirred and heated to reflux, and 1.65 g (10 mmol) of ethyl 2-chloroacetoacetate was added dropwise. Co...
Embodiment 2
[0114] Example 2: Preparation of 2-(3-cyano-4-ethoxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid (compound 2)
[0115] 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid ethyl ester was prepared in the same manner as in Example 1.
[0116] Alkylation: crude ethyl 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-selenazole-5-carboxylate 336 mg (1 mmol), potassium carbonate 138 mg (1 mmol), 5 mg potassium iodide and 2 mL DMF were mixed, and 108 mg (1 mmol) of ethyl bromide was added dropwise under stirring at room temperature: the reaction liquid was heated to 35 °C and reacted for 8 h; after cooling, it was poured into five times the amount of water, and a solid was precipitated. Suction filtration, washing the filter cake with water, and drying to obtain crude ethyl 2-(3-cyano-4-ethoxyphenyl)-4-methyl-1,3-selenazole-5-carboxylate with a yield of 83%. The crude product was directly used in the next reaction without purification.
[0117] Hydrolysis: 364 mg (...
Embodiment 3
[0119] Example 3: Preparation of 2-(3-cyano-4-propoxyphenyl)-4-methyl-1,3-selenazole-5-carboxylic acid (compound 3)
[0120] In addition to using the corresponding alkylating agent in the alkylation step, the same method as in Example 2 was used to prepare 2-(3-cyano-4-propoxyphenyl)-4-methyl-1,3- Selenazole-5-carboxylic acid (compound 3), the total yield is 17% (based on raw material p-hydroxybenzonitrile), its structural formula, 1 H-NMR and MS data are listed in Table-1 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com