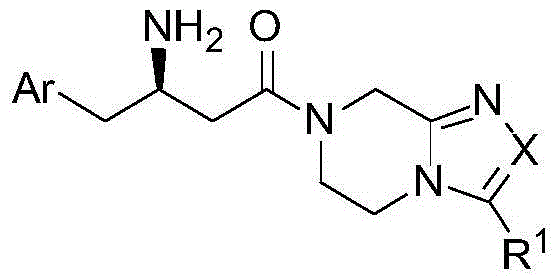
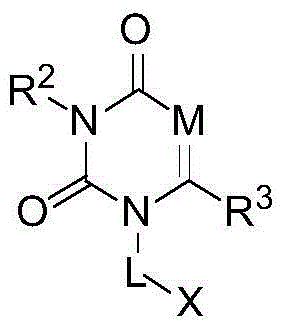
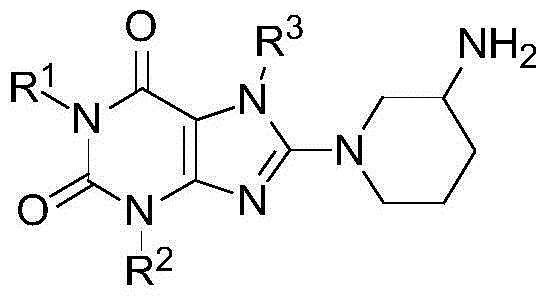
DPP4 inhibitor modified derivative of GLP-1 analogue
A technology of GLP-1 and DPP4, which can be applied in drug combinations, peptide preparation methods, animal/human proteins, etc., and can solve the problems of high clearance rate limiting effectiveness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment one: the synthesis of Palmitoyl-OSu activated lipid
[0086] Weigh 25.64g n-hexadecanoic acid (100mmol), add 13.81g HOSu (120mmol) into 200ml THF, add 24.76g DCC (120mmol) under ice-water bath, react for 1 hour, raise the temperature to room temperature for 3 hours, and filter the reaction solution. The mother liquor was spin-dried, dissolved in DCM, filtered, washed 3 times with saturated sodium bicarbonate, 2 times with pure water, back-extracted 2 times, combined organic phase, dried with anhydrous sodium carbonate, spin-dried, recrystallized with ice ethanol 3 times, filtered, The solid oil pump dried to 31.46g Palmitoyl-OSu activated fat, yield 89%.
Embodiment 2
[0087] Embodiment two: the synthesis of Palmitoyl-Glu-OtBu
[0088] Weigh 10.16g H-Glu-OtBu (50mmol) and 7.95g Na2CO3 (75mmol) into a mixed solution of 50ml water and 50ml THF to dissolve, weigh 17.68g Palmitoyl-OSu (50mmol) into 50ml THF, dissolve and drop Add it to the above mixed solution, react overnight at room temperature, adjust the pH to 7 with 10% dilute hydrochloric acid, remove THF by rotary evaporation, and then adjust the pH to 3. A large white precipitate was obtained which was filtered. The resulting white precipitate was recrystallized from ice ethanol. The solid oil pump was pulled dry to 19.21g Palmitoyl-Glu-OtBu, and the yield was 87%.
Embodiment 3
[0089] Example 3: Synthesis of Palmitoyl-Glu(OSu)-OtBu
[0090] Weigh 8.83g Palmitoyl-Glu-OtBu (20mmol), add 2.76g HOSu (24mmol) into 100ml THF, add 4.95g DCC (24mmol) under ice-water bath, react for 1 hour, warm up to room temperature for 3 hours, and filter the reaction solution. The mother liquor was spin-dried, dissolved in DCM, filtered, washed 3 times with saturated sodium bicarbonate, 2 times with pure water, back-extracted 2 times, combined organic phase, dried with anhydrous sodium carbonate, spin-dried, recrystallized with ice ethanol 3 times, filtered, The solid oil pump is pulled dry to 9.48g Palmitoyl-Glu(OSu)-OtBu activated fat, and the yield is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com