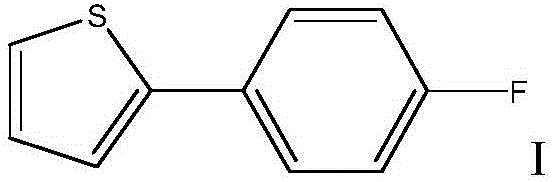
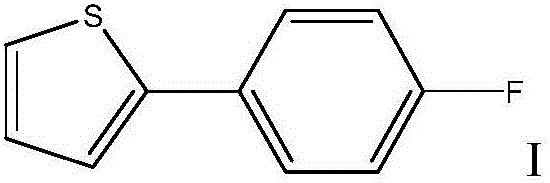
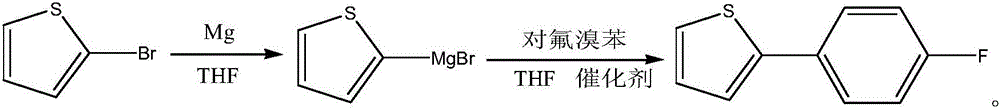Method for preparing Canagliflozin intermediate 2-(4-fluoro-phenyl) thiophene
A technology of fluorophenyl and intermediates, applied in the field of preparation of canagliflozin intermediate 2-thiophene, which can solve the problems of high cost, complex operation industry, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1.1 Format response
[0032] In a 1000ml four-necked flask under nitrogen protection, 14 grams (0.58 mol) of magnesium flakes, 40 grams of THF, and 1 gram of 2-bromothiophene were put in and stirred. At around 30°C, the reaction begins to initiate. (bubbles are generated, and the temperature naturally rises to 35-45°C). The temperature was lowered to 30°C with cold water, and a mixture of 81 g (0.5 mol) of 2-bromothiophene (0.5 mol) and 160 g of THF was started dropwise, and the dropwise addition was completed in about two hours. During the dropping process, the temperature is controlled at about 30°C. After dropping, keep warm at 30°C for 30-60 minutes, take a sample, and the raw material 2-bromothiophene <0.5% is qualified. Finish. Seal and set aside.
[0033] 1.2 Coupling reaction
[0034] In another 1000ml four-neck flask. Under nitrogen protection, 40 grams of THF and 93 grams (0.53 mol) of p-fluorobromobenzene were added. Stir on. Throw in 0.9 g (0.005 mo...
Embodiment 2
[0038] 2.1 Format response
[0039] In a 1000ml four-necked flask under nitrogen protection, 28 grams (0.116 mol) of magnesium flakes, 80 grams of THF, and 2 grams of 2-bromothiophene were put into the flask and stirred. At about 30°C, the reaction starts to occur (bubbles are generated, and the temperature naturally rises to 35-45°C). The temperature was lowered to 30°C with cold water, and a mixture of 162 g (1.0 mol) of 2-bromothiophene (1.0 mol) and 320 g of THF was started dropwise, and the dropwise addition was completed in about two hours. During the dropping process, the temperature is controlled at about 30°C. After dropping, keep warm at 30°C for 30-60 minutes, take a sample, and the raw material 2-bromothiophene <0.5% is qualified. Finish. Seal and set aside.
[0040] 2.2 Coupling reaction
[0041]In another 2000ml four-neck flask. Under nitrogen protection, 40 grams of THF and 187 grams (1.05 mol) of p-fluorobromobenzene were added. Stir on. Throw in 1.8 gr...
Embodiment 2
[0044] Example 2 is actually very similar to the reaction conditions of Example 1. The main reason is that Example 2 doubles the reaction of Example 1, and when the purity is basically close, the yield has a relatively large increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com