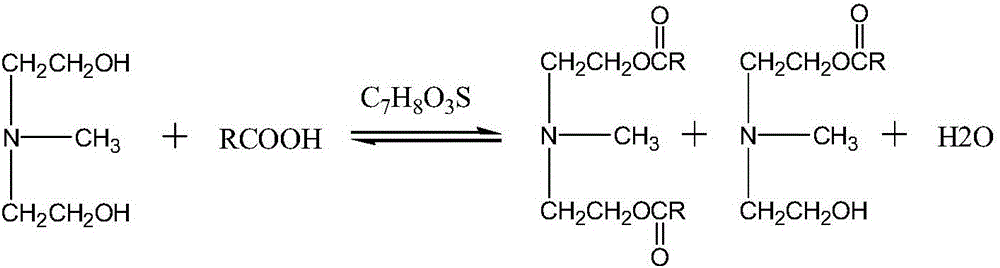Preparation method of novel ester organosilicon quaternary ammonium salt
A technology of organosilicon quaternary ammonium salt and ester group propyltrialkoxysilyl ammonium chloride, which is applied in the field of preparation of ester group organosilicon quaternary ammonium salt, and can solve the problems of low conversion rate of ester amine, high reaction temperature, and reaction Long time and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
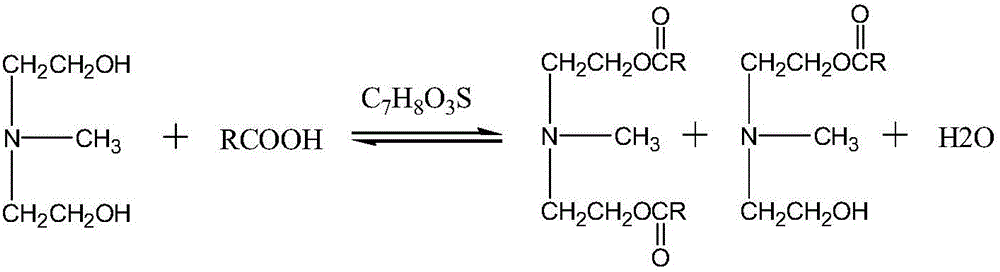
[0011] The present invention provides novel ester group organosilicon quaternary ammonium salt: the synthetic method of methyldiethanolamine laurate mono(bis) ester group propyl trialkoxysilyl ammonium chloride, the method comprises two steps:
[0012] 1) Synthesis of methyldiethanolamine laurate mono(double) ester
[0013] Carry out esterification reaction by N-methyldiethanolamine and lauric acid, take p-toluenesulfonic acid as catalyst;
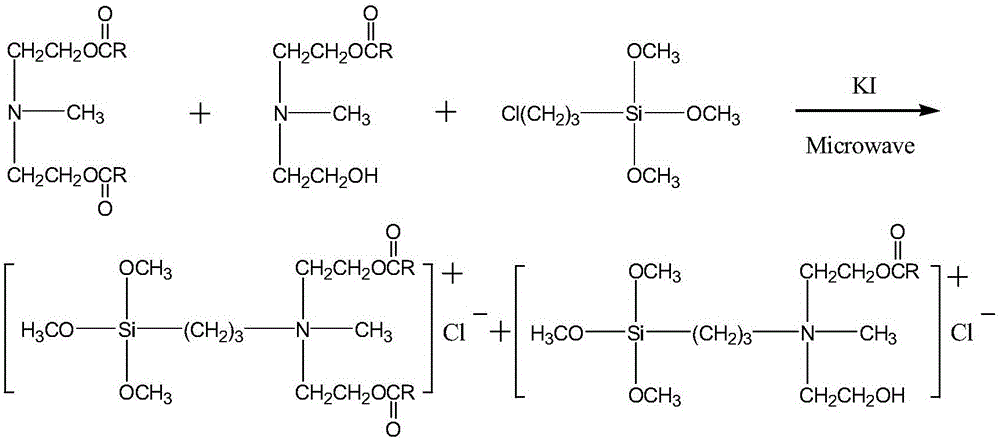
[0014] 2) Synthesis of methyldiethanolamine laurate mono(bis)esteryl propyl trialkoxysilyl ammonium chloride:
[0015] Methyldiethanolamine laurate (double) ester and γ-chloropropyltrialkoxysilane were subjected to quaternization reaction, and methyldiethanolamine laurate was synthesized by microwave method with DMF as solvent and potassium iodide as catalyst. Bis) Ethoxypropyltrialkoxysilyl Ammonium Chloride.
[0016] The γ-chloropropyl trialkoxysilane is γ-chloropropyl trimethoxysilane or γ-chloropropyl triethoxysilane, and the lauric ...
Embodiment 1
[0023] 1. Synthesis of methyldiethanolamine laurate
[0024] Weigh the lauric acid (C 12 h 24 o 2 ), N-methyldiethanolamine (C 5 h 13 NO 2 ), the catalyst p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O), join in the there-necked flask. Of which: lauric acid (C 12 h 24 o 2 ) and N-methyldiethanolamine (C 5 h 13 NO 2 ) in a molar ratio of 1.6:1, p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O) and lauric acid (C 12 h 24 o 2 ) in a mol ratio of 0.008:1, magnetic stirring, and passing N 2 Protection, oil bath heating reaction, reaction temperature 170 ° C, reaction time 6 hours, the conversion rate of lauric acid reaches 99%; the esterification reaction equation is as follows:
[0025]
[0026] R=C in the above formula 11 h 23 ;
[0027] 2. The synthesis of lauric acid methyldiethanolamine mono(double) ester group propyl trimethoxysilyl ammonium chloride will react the product that obtains in the first step, add solvent N successively, N-dimethyl formamide, c...
Embodiment 2
[0032] 1. Synthesis of methyldiethanolamine laurate
[0033] Weigh the lauric acid (C 12 h 24 o 2 ), N-methyldiethanolamine (C 5 h 13 NO 2 ), the catalyst p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O), join in the there-necked flask. Of which: lauric acid (C 12 h 24 o 2 ) and N-methyldiethanolamine (C 5 h 13 NO 2 ) in a molar ratio of 1.6:1, p-toluenesulfonic acid (C 7 h 8 o 3 S·H 2 O) and lauric acid (C 12 h 24 o 2 ) in a mol ratio of 0.008:1, magnetic stirring, and passing N 2 Protection, oil bath heating reaction, reaction temperature 170 ° C, reaction time 6 hours, the conversion rate of lauric acid reaches 99%; the esterification reaction equation is as follows:
[0034]
[0035] R=C in the above formula 11 h 23 ;
[0036] 2. Synthesis of methyldiethanolamine laurate mono(double) ester group propyl triethoxysilyl ammonium chloride
[0037] The product obtained in the first step reaction is sequentially added to the solvent N,N-dimethylformamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com